Chapter 4 The immune response to HIV
Introduction
In marked contrast stands HIV infection. The spread of HIV-1 worldwide represents one of the great challenges to confront host immunity, since the key target is the CD4 T cell lymphocyte, infection and depletion of which severely undermines effective immune responses. As a result, most people who become infected and remain untreated will ultimately succumb to one or more of the large variety of infectious organisms that humans are confronted with on a daily basis. By 2010, there was an estimated 2.4–2.9 million people becoming newly infected with HIV, with 1.8 million dying of AIDS-related causes (http://www.unaids.org, 2011 Global Report).
Acquired Immunity to Viral Infections
The MHC in humans is known as the human leukocyte antigen (HLA) system and is one of the most polymorphic proteins in the human population. The uniqueness of individuals is partly defined by HLA, where each person has a defined HLA type consisting of pairs of inherited genes. As the sole function of class I and II HLA is to present processed pathogen-derived peptides, or epitopes, to circulating T cells, possible aberrant T cell function and recognition of self, as in autoimmunity, will thus involve the HLA. HLA class I molecules are co-dominantly expressed on antigen presenting cells, and they play an important role in regulating the fitness of the immune system through a process of selecting and presenting immunogenic peptides to CD8 T cells by TcR recognition (Fig. 4.1A). HLA alleles are X-linked and inherited in pairs (heterozygous), and it is noteworthy that in HIV infection, individuals who are homozygous for one or more alleles (inheriting the same HLA allele from both parents) progress more rapidly to AIDS than heterozygotes [1]. Additionally, HLA-B is more polymorphic than HLA-A and HLA-C and the influence of HLA-B is known to have the strongest impact in HIV set point, which is strongly predictive of the rate of progression [2] when compared to HLA-A and HLA-C molecules [3, 4]. The manner by which epitopes are processed and bound by the HLA molecule is highly specific and governed by certain rules associated with the binding motif structures of each HLA and in the correct orientation will be recognized by activated CD8 cytotoxic T lymphocytes (Fig. 4.1A). Sequence changes can occur at anchor positions of targeted epitopes and reduce or interfere with peptide binding to the restricting HLA class I molecule. Moreover, amino acid changes within or immediately adjacent to CD8 T cell epitopes can impede intracellular antigen processing or directly modify the structural interaction between the epitope of HLA class I complex and the TcR of the corresponding CD8 T cells. The ability of viruses to acquire sequence mutations resulting in the loss of recognition by HIV-1-specific CD8 T cells poses a major hurdle for current vaccine efforts.
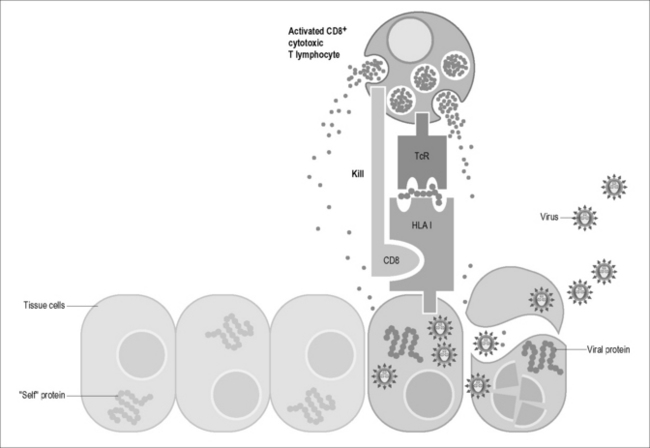
Figure 4.1A Cells infected by viruses or intracellular bacteria are detected and destroyed by CD8 cytotoxic T lymphocytes. CD8 cytotoxic T lymphocytes are activated in the secondary lymphoid organs and migrate to sites of inflammation where they scan cell surfaces with their T cell receptors (TcR) for recognition of foreign peptide antigens displayed on cell surface HLA class I molecules. HLA class I receptors are constitutively expressed on the surface of all cells except erythrocytes. CD8 cytotoxic T lymphocytes destroy target cells by releasing granzymes and perforin and cell-degrading molecules (with permission from Immunopaedia, http://www.immunopaedia.org).
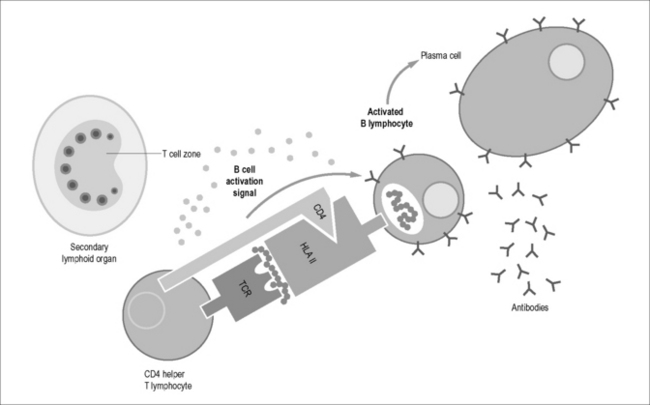
HLA class II molecules have a more restricted distribution and are expressed only on specific cell types and on T cells after activation. Classically, CD4 T cells provide “help” to the immune response by liberating a series of cytokines important for coordinating cellular activity and inducing activated B cells to become antibody-secreting plasma cells (Fig. 4.1B). First identified in murine models, the multitudinous number of cytokines have been organized into a network model of Th1, Th2, Treg, and Th17 cells. A Th1-type response consists of CD4 T cells liberating a profile of cytokines that direct T cell immunity and involves IL-1, IL-2, IL-6, IL-12, IL-15, TNF-α, and IFN-γ, for example. A Th-2-type response consists of CD4 T cells liberating a profile of cytokines that directs humoral immune responses and is involved in switching on B cell immunity. These cytokines include, among others, IL-4, IL-5, and IL-10. A Th17-type response consists of an IL-17A, IL-17F, and IL-22 profile and is involved in conferring protection against bacteria, fungi, and mycobacteria [5] and to play a role in mucosal defense in the gut. Tregs are CD4+CD25hiFoxP3+ and have been shown to downregulate the activation and proliferation of T cells [5]. A balance exists between pro- and anti-inflammatory immune responses imparted by these CD4 subsets for maintaining the integrity and homeostatic balance of cells in the host. Recently, T helper follicular cells that are involved in the development of antibody-producing plasma cells in the germinal centers of lymphoid tissue have been described [6].
How do these cells fit together in healthy humans? CD8 T cells make up the smaller proportion of CD3 T cells and are involved in protecting the host from invading pathogens. These cells function by killing virally infected cells, which are marked by the surface expression of HLA class I molecules that present virus-derived epitopes that are typically 8-11 amino acids in length (as described above, Fig. 4.1A). These CD8 T cells function with the help of CD4 T helper cells. The killing potential of CD8 T cells is through either perforin/granzyme or Fas–Fas-L interactions and erupted and effete infected cells are engulfed and processed by dendritic cells; virally derived epitopes are presented via cross-presentation [7] by class II HLA molecules and drive CD4 T cell responses. Typically most (99%) expanded viral antigen-specific T cell effector clones induced in the acute phase of infection will die through apoptosis as the immune response wanes, leaving a small residual population of T effector memory cells that migrate to non-lymphoid tissues or T central memory (TCM) cells that recirculate through the lymphatic system and blood circulation and can be rapidly reactivated upon secondary exposure to viral antigens.
Immune Response to HIV-1 infection
Is HIV a disease of the mucosal immune system? A number of studies have highlighted the importance of the mucosa in HIV pathogenesis and it is now increasingly being recognized as a disease of the mucosal immune system [8], where vaginal and rectal mucosa are the predominant sites of HIV entry and the gut-associated lymphoid tissue (GALT) is the site of initial HIV replication. During the early phase of simian immunodeficiency virus (SIV) and HIV infection, there is a rapid and widespread massive depletion of activated mucosal CD4 T cells at mucosal sites and this occurs before significant depletion in blood and lymph nodes [9]. Recent evidence confirms that the level of CD4 T cell depletion is far higher than at first anticipated, with 60–80% of memory CD4 T cells depleted during early infection. Thus, within the first few weeks of HIV infection, the virus targets the mucosal immune system and dramatically depletes the CD4 T cells at this site. It has also been shown that HIV targeting of activated CD4 T cells in mucosal tissues persists throughout infection, and not just in acute infection as previously thought. Mucosal tissues are likely to be a major source of viral replication, persistence, and continual CD4 T cell loss in HIV-infected individuals. Reduced CD4 T cell frequencies during chronic HIV infection have also been shown in other mucosal sites such as rectal mucosa [10], male genital tract [11], female genital tract [9], and lung mucosa [12].
Figure 4.2 shows some of the local factors in the mucosal microenvironment that may facilitate HIV replication in mucosal tissues independently from blood: (i) the localized cytokine milieu; (ii) differing inflammatory signals; and (iii) the presence of different immune cell types in these distinct compartments. These factors highlight mucosal sites as critically important in the context of not only understanding HIV pathogenesis but also in terms of being able to possibly dampen or correct the imbalance of pro-inflammatory signals in potential therapeutic or preventive modalities.
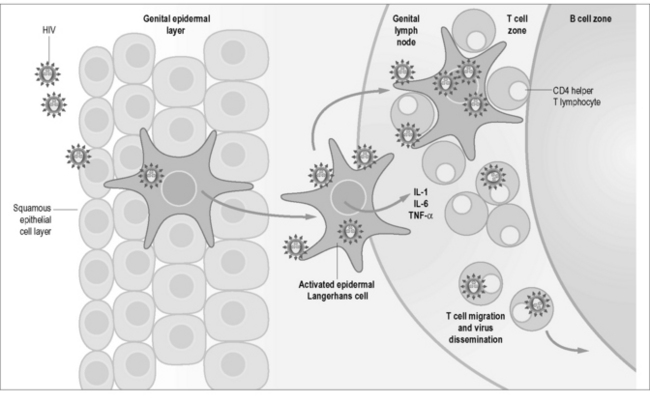
Figure 4.2 Epidermal Langerhans cells are a subset of dendritic cells found in the squamous epithelium of the female vagina and male inner foreskin and are the first immune cells to contact HIV during heterosexual contact. They express surface CD207 (langerin) that capture virus by binding to gp120 and induces internalization and degradation of virus. Activated cells migrate to draining lymph nodes for antigen presentation to CD4 T lymphocytes, which can also become infected by surface-bound virus. Langerhans cells also express CD4 and CCR5 and can become infected. Activated Langerhans cells produce pro-inflammatory cytokines IL-1, IL-6, and TNF-α that can cause fever in acute infection (with permission from Immunopaedia, http://www.immunopaedia.org).
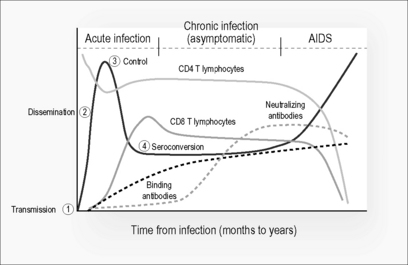
During acute HIV infection, there appears to be a hierarchy of systemic immune responses that occur. Figure 4.3 shows a composite schema of the known sequence of immunological responses that occur during infection. After viral transmission (1), where there appears to be a selection of single strain variants at the mucosa [13], there is dissemination (2) of the virus to the lymphoid tissue [14] during the acute phase of infection. Within days after viral transmission, viremia peaks and the downward slope is thought to be a result of a robust cellular immune response leading to initial control (3) of virus [15]. Natural history studies have shown that viral set point is achieved within 6 months of infection and is prognostic of disease outcome, where high levels of viremia are associated with a more rapid course of infection leading to AIDS. It is noteworthy that seroconversion (4), by the detection of anti-Gag binding antibodies, occurs after peak viremia and that detection of neutralizing antibodies occurs only after approximately 3 months post transmission.
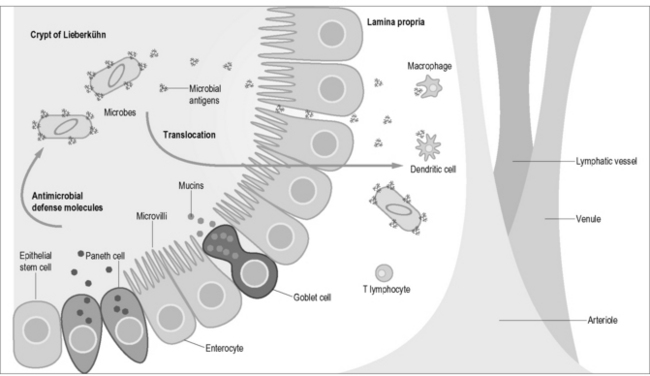
In addition to virus-specific CD8 T cells, CD4 T cells appear to be critical for immune control. Animal models of chronic viral infections established that virus-specific CD4 T cells play an essential role in maintenance of effective immunity (reviewed in Day and Walker [16]), and the immune response to HIV appears to follow these same requirements. The detection of enhanced proliferation of anti-HIV-specific CD4 T cells in individuals who maintain long-term control of HIV replication [17] and in patients treated for acute infection with potent antiretroviral therapy [17–19] suggest that the function of these cells is central to influencing viral set point and for controlling virus. As discussed, a large number of CD4 T cells are infected in the gut and that the bulk of the CD4 T cell pool resides within lymphoid tissue around the gastrointestinal tract. Direct killing of CCR5 CD4 T cells within the gut [20, 21] and memory CD4 cells in multiple tissues [22] by HIV has been postulated as the main mechanism for CD4 T cell depletion during SIV infection in monkeys, and extrapolations to HIV-induced depletion of CD4 T cells within the human gut have also been made [23]. Figure 4.4 shows the potential scenario of microbial translocation from the gut lumen. Studies of HIV/AIDS pathogenesis have long-focused on the role of CD4 T-cell depletion as a key marker of disease progression [24]. The pathogenesis of HIV infection is now characterized by CD4 T cell immunodeficiency in the context of generalized immune activation and dysregulation, with massive memory CD4 T cell infection and depletion during acute infection. This is followed by gradual loss of remaining CD4 T cells caused by persistent immune hyperactivation. Activation of CD4 T cells results in increased target cells for the virus, excessive apoptosis of uninfected T cells, generalized immune dysfunction, and impaired ability to control HIV replication.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree