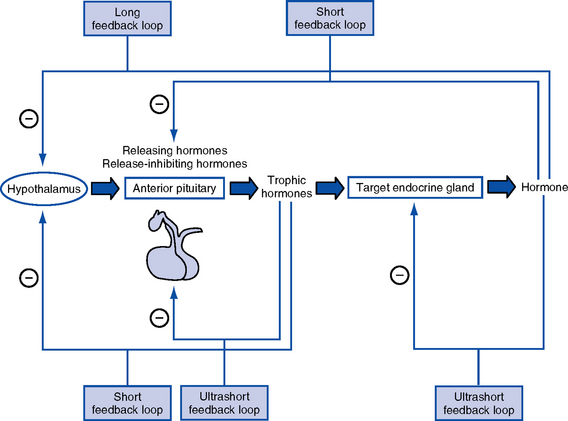
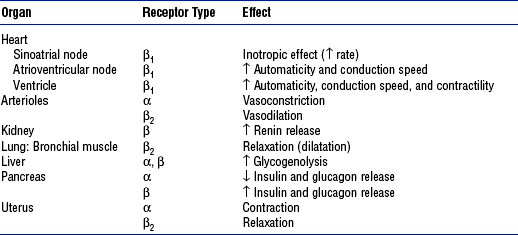
CHAPTER 6 a. Hormones are molecules that are synthesized and secreted by specialized cells and released into the blood, exerting biochemical effects on target cells away from the site of origin b. Hormones control metabolism, transport of substances across cell membranes, fluid and electrolyte balance, growth and development, adaptation, and reproduction 2. Chemically categorized by physiologic action a. Peptide or protein hormones: Vasopressin (antidiuretic hormone [ADH]) thyrotropin-releasing hormone (TRH), insulin, growth hormone (somatotropin [GH]), follicle-stimulating hormone (FSH), luteinizing hormone (LH), corticotropin (adrenocorticotropic hormone [ACTH]), calcitonin b. Steroids: Glucocorticoids (cortisol), mineralocorticoids (aldosterone), estradiol, progesterone, testosterone c. Amines and amino acid derivatives: Norepinephrine, epinephrine, triiodothyronine (T3), thyroxine (T4) a. Specificity of hormone action is determined by the presence of a specific hormone receptor on or in the target cell b. Receptors distinguish hormones from each other and translate the hormonal signal into a cellular response c. The hormone-receptor complex initiates intracellular events that lead to the biologic effects of the hormone acting on the target cell 4. Mechanisms of hormone action a. Activation of cyclic adenosine monophosphate (cAMP): Thyrotropin (thyroid-stimulating hormone [TSH]), ACTH, parathyroid hormone (PTH), and ADH b. Activation of genes: Steroid hormones and gonadal hormones 5. Feedback control of hormone production (Figure 6-1) 1. Location: Base of the skull in the sphenoid bone; connected to the hypothalamus by the pituitary stalk (infundibulum), which links the nervous and endocrine systems a. Anterior lobe (adenohypophysis—75% of gland): Hormones are controlled by hypothalamic releasing or inhibiting hormones in response to stimuli received in the central nervous system b. Posterior lobe (neurohypophysis—25% of gland) 3. Anterior pituitary hormones (a) Stimulation: GH-releasing hormone (GRH) in response to physical and/or emotional stress, starvation, hypoglycemia, other protein-depleted states (b) Inhibition: Somatostatin from the hypothalamus, postprandial hyperglycemia, and pharmacologic doses of corticosteroids (a) Increases rate of protein synthesis (c) Decreases protein catabolism (d) Decreases carbohydrate use (e) Stimulates bone and cartilage growth (f) Works with insulin, thyroid hormones, and sex steroids to promote growth iii. Disorders resulting from dysfunction: Not of significance in critical care (a) Stimulation: Corticotropin-releasing hormone (CRH) in response to physical or emotional stress, trauma, hypoglycemia, hypoxia, surgery, decreased plasma cortisol levels (b) Inhibition: Increased plasma cortisol levels exert negative feedback on CRH and thus ACTH; stress can overcome this negative feedback ii. Physiologic activity: Production and release of adrenocortical hormones (glucocorticoids, adrenal androgens, and mineralocorticoids) iii. Disorders resulting from dysfunction (a) Stimulation: TRH in response to low concentration of thyroid hormones (b) Inhibition: Somatostatin from the hypothalamus, increased thyroid hormone levels (a) Increases synthesis of thyroid hormones (b) Releases stored thyroid hormones (c) Stimulates iodide uptake into thyroid cells (d) Increases size, number, and secretory activities of thyroid cells iii. Disorders resulting from dysfunction: See Thyroid Gland d. Other anterior pituitary hormones under hypothalamic control 4. Posterior pituitary hormones (a) Stimulation: Increase in plasma osmolality, hypoxia, reduction in blood volume or blood pressure (a) Increases water permeability in renal collecting duct epithelial cells, thereby controlling extracellular fluid osmolality (b) In pharmacologic amounts, constricts arterioles to increase blood pressure iii. Disorders resulting from dysfunction 1. Location: Immediately below the larynx laterally and anterior to the trachea 2. Composition: Two lobes connected by an isthmus 3. Regulation of secretion (thyroid hormones) a. Stimulation: TSH stimulates thyroid hormone release, which is regulated by TRH from the hypothalamus; decreased levels of thyroid hormones stimulate the release of TSH and TRH b. Inhibition: Elevated levels of thyroid hormones inhibit TSH and TRH a. Increases the metabolic activity of cells, which results in increased oxygen consumption, increased rate of chemical reactions, and heat production b. Stimulates carbohydrate, fat, and protein metabolism c. Works with insulin, GH, and sex steroids to promote growth d. Critical for fetal neural and skeletal system development (intrauterine hypothyroidism causes cretinism) e. Positive chronotropic and inotropic effects on the heart f. Required for a normal hypoxic and hypercapnic drive in respiratory centers h. Increases metabolism and clearance of steroid hormone and insulin 5. Disorders resulting from dysfunction a. Thyroid enlargement (goiter) b. Excess: Hyperthyroidism (chronic), thyroid storm (acute) c. Deficiency: Hypothyroidism (chronic), myxedema coma (acute) 6. Thyrocalcitonin (calcitonin) 1. Location: Four glands on the posterior surface of the thyroid gland 2. Composition: Chief cells release PTH a. Stimulation: Decrease in serum calcium level b. Inhibition: Increase in serum levels of calcium and vitamin D metabolites, hypermagnesemia, and hypomagnesemia i. Increases renal tubular reabsorption of calcium and magnesium ii. Decreases renal tubular reabsorption of phosphate and bicarbonate iii. Stimulates the formation of the fat-soluble form of vitamin D b. Gastrointestinal tract: Increases calcium absorption 5. Disorders resulting from dysfunction 1. Location: Retroperitoneal, superior to the kidney 2. Composition: Two separate endocrine tissues that produce distinct hormones a. Glucocorticoids (cortisol is the major hormone) (a) Stimulation: ACTH (diurnal variation—increased 1 hour after awakening, incidence of myocardial infarction increased in the morning) (b) Inhibition: Cortisol exerts negative feedback on the anterior pituitary and hypothalamus (1) Decreases protein stores and protein synthesis in all cells except liver cells (2) Increases protein catabolism (d) Increases tissue responsiveness to other hormones, such as glucagon and the catecholamines iii. Disorders resulting from dysfunction b. Mineralocorticoids (aldosterone is the major hormone) 4. Medullary hormones: Epinephrine and norepinephrine a. Regulation of secretion: Stimulated by fear, anxiety, pain, trauma, fluid loss, hemorrhage, extremes in temperature, surgery, hypoxia, hypoglycemia, hypokalemia, hypernatremia, hypotension b. Physiologic activity (Table 6-1) c. Disorders resulting from dysfunction 1. Location: Lies transversely behind the peritoneum and stomach 2. Composition: Exocrine and endocrine components. Endocrine functions originate from the islet cells, which constitute less than 2% of the total pancreatic volume; 65% of the islet cells are beta cells, which produce insulin. Glucagon is produced by the alpha cells; somatostatin and gastrin are produced by the delta cells. i. Stimulation: Increases in blood glucose, gastrin, secretin, cholecystokinin, and gastrointestinal hormone levels, and β-adrenergic stimulation ii. Inhibition: α-adrenergic effects of somatostatin, catecholamines, and drugs, including diazoxide, phenytoin, and vinblastine iv. Works with thyroid hormones, the sex steroids, and GH to promote growth c. Disorders resulting from dysfunction ii. Deficiency: Diabetes mellitus (a) Type 1: Absolute deficiency of insulin due to islet cell antibodies; genetic link, autoimmune disorder (b) Type 2: Relative deficiency of insulin caused by decreased sensitivity of receptors to insulin, decreased production, premature destruction of insulin or receptors, and/or hyperinsulinemia; polygenetic etiologies, dietary link i. Increases blood glucose via glycogenolysis and gluconeogenesis iii. Increases amino acid transport to the liver and the conversion of amino acids to glucose precursors iv. Is a major insulin-antagonistic hormone v. Critical hormone in the recovery from insulin-induced hypoglycemia c. Deficient glucagon production is thought to play a role in defective glucose counterregulation in insulin-induced hypoglycemia in type 1 diabetes mellitus d. Available as a pharmacologic agent to correct insulin-induced hypoglycemia (all diabetics should have a readily available source) i. Presence of pathophysiologic processes that can result in endocrine dysfunction (a) Adrenal gland hypoperfusion (b) Infection, inflammation, autoimmune processes (c) Neoplasms and exposure to the chemotherapeutic agents and radiotherapy used to treat the neoplasms ii. Pregnancy, postpartum state iii. Presence of preexisting chronic endocrine disorder (diagnosed or undiagnosed) iv. Poor compliance with pharmacologic therapy for a preexisting endocrine disorder v. Presence of an unrelated critical illness in a patient with a preexisting chronic endocrine disorder vi. Positive family history of an endocrine disorder viii. Indicators of altered health patterns (1) Personality changes, lethargy, emotional lability, attention span deficit, memory impairment (3) Changes in level of consciousness (4) Depression, paranoia, delusions, delirium (5) Verbalizations that indicate lack of knowledge or misconceptions regarding self-care management (e) Sleep and rest: Restlessness, inadequate sleep (1) Discord in previously stable relationships (2) Physical and emotional inability to engage in usual role activity (h) Coping and stress tolerance (i) Health perception and health management: Evidence of noncompliance with the prescribed medical regimen b. Family history: Endocrine disorders in other family members i. Elderly persons may be at special risk for the development of an endocrine crisis because of changes associated with aging and a diminished thirst mechanism ii. Economically disadvantaged persons may be at risk for the development of an endocrine crisis because many of the regimens for treating chronic endocrine disorders are costly and necessitate regular medical follow-up iii. Teenagers with poor compliance with a prescribed medical regimen, particularly diabetic patients, are at increased risk of crisis 2. Nursing examination of patient (a) Excessive or diminutive stature (b) Fat distribution in relation to gender and maturational level (c) Mobility, tremor, hyperkinesis (d) Scars, especially in the neck area (e) Hair distribution and texture relative to gender and maturational level (i) Presence of medical alert identification (j) Hydration status of oral cavity (k) Periorbital edema, ptosis, eye protrusion, stare, dry eyes (l) Unusual pigmentation, temperature, turgor, striae, or thinning of the skin ii. Palpation: Enlarged or nodular thyroid gland, often painful iii. Percussion: Abnormal deep tendon reflexes (may be hyperreflexic or hyporeflexic) (a) Neck: Bruits over the thyroid gland (b) Heart: Distant heart sounds, third heart sound (due to pericardial effusion, heart failure) (c) Blood pressure: Hypotension, hypertension (d) Heart rate and rhythm disturbances (e) Altered respiratory pattern (g) Pericardial and/or pleural friction rub (due to effusion) 3. Appraisal of patient characteristics i. Level 1—Minimally resilient: 88-year-old female admitted in a diabetic coma with concomitant antibiotic-resistant bacterial pneumonia ii. Level 3—Moderately resilient: 14-year-old male admitted in diabetic ketoacidosis following an episode of the flu; no other medical conditions iii. Level 5—Highly resilient: 57-year-old man with a blood glucose level of 50 mg/dl who reports feeling sweaty, agitated, and slightly disoriented following 3 days of vomiting with the flu i. Level 1—Highly vulnerable: 79-year-old male with a history of myocardial infarction and subsequent congestive heart failure who develops diabetes insipidus following head trauma after a motor vehicle accident; fluid replacement causes cardiac deterioration ii. Level 3—Moderately vulnerable: 49-year-old female who develops nephrogenic diabetes insipidus following repeated episodes of pyelonephritis with scarring iii. Level 5—Minimally vulnerable: 44-year-old male following transsphenoidal removal of a pituitary tumor who develops diabetes insipidus but remains hemodynamically stable and responds immediately to administration of vasopressin i. Level 1—Minimally stable: 38-year-old female who attempts suicide by ingestion of excessive amounts of thyroid hormone; arrives at the unit with severe tachycardia, hypotension, and a temperature of 105° F (40.6° C), in a coma ii. Level 3—Moderately stable: 44-year-old female who comes for treatment with tachycardia and a blood pressure of 90/60 mm Hg after restarting thyroid hormone therapy with a new formulation iii. Level 5—Highly stable: 32-year-old female with hyperthyroidism as evidenced by abnormal laboratory test results who responds well to propranolol and propylthiouracil and who is scheduled for thyroidectomy i. Level 1—Highly complex: 88-year-old female admitted in hyperosmolar, nonketotic coma. Patient has a history of malnutrition and chronic obstructive pulmonary disease. She lives alone, has mobility impairments, and has no family nearby. Has Medicare insurance only. ii. Level 3—Moderately complex: 44-year-old male admitted in diabetic ketoacidosis following inability to obtain insulin. Patient recently became unemployed and lost health insurance and prescription coverage. iii. Level 5—Minimally complex: 79-year-old male with newly diagnosed diabetes who has a blood glucose level of 200 mg/dl and a hemoglobin A1C fraction of 8.6%. Patient shows cardiovascular and neurologic stability. Has good family support and insurance. Is well educated. i. Level 1—Few resources: Patient with newly diagnosed diabetes who has no insurance and no family, is unemployed, is new to the area, and is homeless ii. Level 3—Moderate resources: Patient with newly diagnosed diabetes who has Medicare coverage and a niece who lives an hour away and who currently resides in an assisted living facility iii. Level 5—Many resources: Patient with newly diagnosed diabetes who has insurance and prescription coverage. Patient is well educated and has strong family support. Independent in care and finances. i. Level 1—No participation: 48-year-old female admitted in myxedema coma following thyroidectomy. Patient did not start thyroid replacement therapy after surgery. No family nearby. ii. Level 3—Moderate level of participation: 68-year-old female admitted with bradycardia, anemia, and fatigue who confesses to having stopped thyroid hormone replacement therapy because she couldn’t afford to visit her physician for a new prescription iii. Level 5—Full participation: A 14-year-old male who is treated successfully for diabetic ketoacidosis and who, with his family, requests assistance in learning more about his disease and its management
The Endocrine System
SYSTEMWIDE ELEMENTS
Pituitary Gland
Thyroid Gland
Parathyroid Glands
Adrenal Glands
Pancreas
PATIENT ASSESSMENT
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Endocrine System
Get Clinical Tree app for offline access