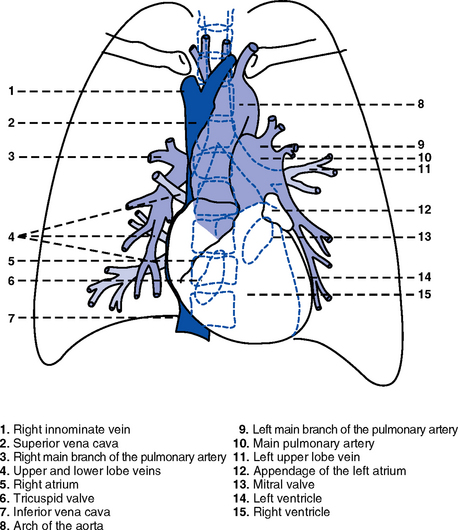
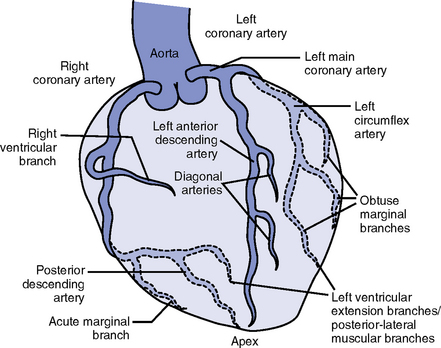
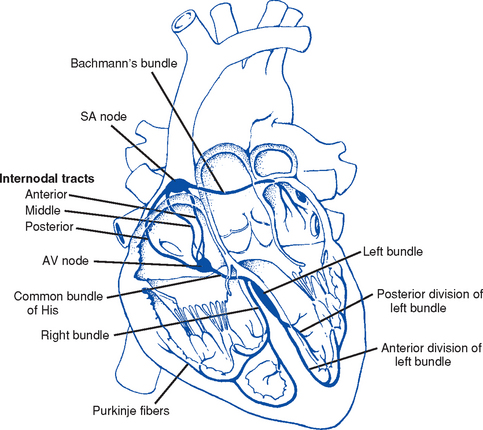
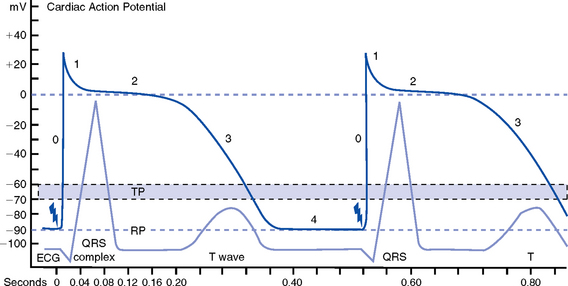
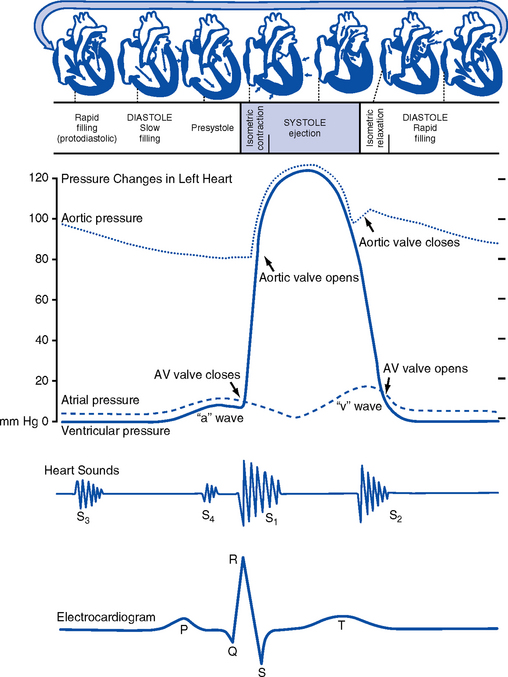
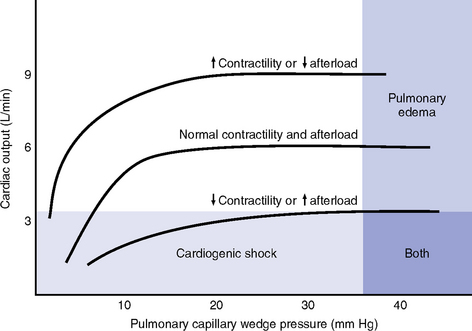
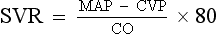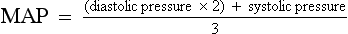CHAPTER 3 1. Heart (Figures 3-1 and 3-2) a. The heart lies in the mediastinum, on and to the left of the midline b. Its long axis is oriented from the right shoulder blade to the left upper quadrant of the abdomen c. The base (top wide area) of the heart (atria and great vessels) is located diagonally at the second intercostal space, right and left sternal borders d. The apex, or tip, of the heart (junction of the ventricles and ventricular septum) is usually located at the fifth intercostal space, on the left midclavicular line a. Pericardium: Fibrous sac surrounding the heart and containing small amounts (15 to 50 ml) of pericardial fluid. This lubricated space protects the heart from friction, allowing it to easily change volume and size during contractions. The pericardium also keeps heart muscle anchored within the mediastinum. b. Epicardium: Outer surface of the heart (includes epicardial coronary arteries, autonomic nerves, adipose tissue, lymphatics) c. Myocardium: Muscular, contractile portion of the heart. Muscle fibers wrap around the heart in multiple, interlacing layers. d. Endocardium: Inner surface of the heart e. Papillary muscles: Myocardial structures extending into the ventricular chambers and attaching to the chordae tendineae f. Chordae tendineae: Strong tendinous attachments from the papillary muscles to the tricuspid and mitral valves; prevent prolapse of the valves into the atria during systole a. Atria: Thin-walled, low-pressure chambers i. Right and left atria act as reservoirs of blood for their respective ventricles ii. Right atrium (RA), located above and to the right of the right ventricle, receives systemic venous blood via the superior vena cava and inferior vena cava, and venous blood from the heart via the coronary sinus iii. Left atrium (LA), superior and posterior to the other chambers, receives oxygenated blood returning to the heart from the lungs via the pulmonary veins iv. When the mitral and tricuspid valves open, there is rapid filling of blood passively from atria into ventricles (about 80% to 85% of total filling) v. At the end of diastole, atrial contraction (“atrial kick”) forcefully adds 15% to 20% more to the ventricular volume b. Ventricles: Major “pumps” of the heart i. Right ventricle (RV) is anterior under the sternum (a) Thin-walled, low-pressure system (b) Contracts and propels deoxygenated blood into the pulmonary circulation via the pulmonary artery (the only artery in the body that carries deoxygenated blood) ii. Left ventricle (LV) is the main “pump”: Conical (ellipsoid) structure behind and to the left of the RV (a) Thick-walled, high-pressure system (b) Squeezes and ejects blood into the systemic circulation via the aorta during ventricular systole iii. Interventricular septum is functionally more a part of the LV than of the RV. It forms the anterior wall of the LV. Its curved shape protrudes into the RV cavity. a. Atrioventricular (AV) valves i. Location and structure: Situated between the atria and the ventricles (tricuspid valve on the right, mitral valve on the left) (a) Tricuspid valve is composed of three leaflets: The large anterior leaflet, and the two smaller posterior and septal leaflets (b) Mitral valve is composed of two leaflets: The long, narrow posterior (mural) leaflet (like a toilet seat) and an oval anterior (aortic) leaflet (like a toilet lid) ii. Function: These are one-way “check” valves that permit unidirectional blood flow from the atria to the ventricles during ventricular diastole and prevent retrograde flow during ventricular systole (a) Pulmonary valve is situated between the RV and the pulmonary artery. It consists of three semilunar cusps that attach to the wall of the pulmonary trunk. (b) Aortic valve is situated between the LV and aorta. It consists of three slightly thicker valve cusps, the bases of which attach to a valve annulus (fibrous ring). ii. Function: Permit unidirectional blood flow from the outflow tract during ventricular systole and prevent retrograde blood flow during ventricular diastole (a) With ventricular systole, the valves open when the respective ventricle contracts and pressure is greater in the ventricle than in the artery (b) After ventricular systole, pressure in the artery exceeds pressure in the ventricles. This and retrograde blood flow cause the valve to close. (c) Second heart sound (S2) is produced when the aortic (A2) and pulmonic (P2) valves close. A2 is normally the initial and major component of S2. 5. Coronary vasculature (Figure 3-3) i. Two main arteries branch off at the base of the aorta, supplying blood to the heart ii. Right coronary artery (RCA) (a) Originates behind the right coronary cusp of the aortic valve iii. Left coronary artery (LCA) (a) Left main coronary artery (LMCA): Branches into the left anterior descending and circumflex arteries (b) Left anterior descending (LAD) artery (1) Supplies the anterior two thirds of the interventricular septum and the anterior wall of the LV (2) Branches include diagonals (two to six other diagonals may be present), septal perforators (three to five other perforators may be present) (c) Left circumflex (LCX) artery also branches from the LMCA c) Inferior-posterior LV in 10% of hearts d) Lateral posterior surface of the LV via the obtuse marginal branches (OMBs) e) Posterior descending artery arises from the LCX artery in 15% of hearts (see description under RCA) (2) Branches of the LCX include the OMBs (may be one to three), which supply the lateral wall of the LV and occasionally the posterior lateral muscular branch iv. Coronary collaterals: Potential vascular connections between the RCA and LCA exist i. Return deoxygenated blood to the RA, mostly through the coronary sinus ii. Follow paths similar to those of the arteries; have no valves i. Coronary vascular reserve: Coronary circulation has the ability to increase flow to meet added needs up to approximately six times normal ii. Coronary blood flow is about 70 to 90 ml/min iii. The heart uses most of the oxygen available in the coronary circulation; little oxygen reserve exists iv. Most of coronary blood flow is in diastole, because in systole, coronary artery blood flow usually decreases due to ventricular compression and contraction v. Coronary blood flow is reduced by 6. Neurologic control of the heart a. Autonomic nervous system: Influences contractility, depolarization-repolarization, and rate of conductivity i. Sympathetic stimulation: Norepinephrine release is the main impetus of stimulation to the heart; its two effects include the following: ii. Parasympathetic stimulation: Occurs via the tenth cranial (vagus) nerve. Acetylcholine release is the main parasympathetic impetus to cardiac effects. iii. Ventricles have mainly sympathetic innervation and only sparse vagal innervation iv. Parasympathetic influences normally predominate in the conducting system (SA node, AV node) b. Chemoreceptors: Afferent receptors located in the carotid and aortic bodies. Sensitive to changes in partial pressure of oxygen, partial pressure of carbon dioxide, and pH, causing changes in heart rate and respiratory rate via stimulation of vasomotor center in the medulla. c. Baroreceptors: Stretch receptors in the heart and blood vessels that respond to pressure and volume changes 7. Cardiac muscle microanatomy and contractile properties: See Box 3-1 for key elements 8. Anatomy of the cardiac conduction system (Figure 3-4) i. Normal pacemaker of the heart, possessing the fastest inherent rate of automaticity (approximately 70 beats/min) ii. Located in the right superior wall of the RA at the junction of the superior vena cava and the RA b. Internodal atrial conduction i. Impulse is conducted from the SA node through the RA and LA musculature to the AV node ii. Although the atria do not have specialized high-speed conduction tracts comparable to the ventricular bundles and fascicles, there are preferred conduction pathways (e.g., Bachmann’s bundle, which conducts impulses from the SA node to the LA) i. Delays the impulse from the atria before it goes to the ventricles. This allows time for both ventricles to fill before ventricular systole. ii. Inherent rate of automaticity is approximately 40 beats/min iii. Located in the right interatrial septum, above the tricuspid valve’s septal leaflet d. Bundle of His: Arises from the AV node and conducts the impulse to the bundle branch system. The bundle of His is close to the annulus of the tricuspid valve. e. Bundle branch system: Pathways that arise from the bundle of His and branch at the top of the interventricular septum i. Right bundle branch is the smaller, direct continuation of the bundle of His. It transmits the impulse down the right side of the interventricular septum to the RV myocardium. ii. Left bundle branch is the larger branch from the bundle of His. It transmits the impulse to the septum and the LV. The left bundle branch divides into three parts: i. Arises from the distal portion of the bundle branches, forming networks on the ventricle’s endocardial surface ii. Transmits the impulse into the subendocardial and myocardial layers of both ventricles iii. Provides for depolarization of the myocardium (from endocardium to epicardium) iv. Ventricles have their own inherent rate of automaticity of approximately 20 to 30 beats/min a. Electrophysiologic properties of cardiac muscle cells i. Excitability: Ability to depolarize and form an action potential when sufficiently stimulated ii. Automaticity: Ability to generate an impulse without an outside stimulus iii. Conductivity: Ability to conduct an electrical impulse to neighboring cells, spreading the impulse throughout the heart to achieve total depolarization iv. Refractoriness: Temporary inability of the depolarized cell to become excited and generate another action potential b. Resting membrane potential (RMP): Electrical charge of cardiac muscle cell at rest. Cell ions consist primarily of sodium, potassium, and calcium. i. Sodium ion concentration is greater outside the cell ii. Potassium ion concentration is greater inside the cell c. Depolarization: Change in the electrical charge of a stimulated cell from negative to positive by the flow of ions across the cell membrane. Sodium moves into the cell, potassium moves out. d. Repolarization: Recovery or recharging of a cell’s normal polarity. Sodium moves back out of the cell, potassium moves into the cell. The cell recovers its negative charge. e. Threshold potential: The electric voltage level at which cardiac cells become activated and produce an action potential, which leads to muscular contraction f. Stimulation of myocardial cells i. Stimulus may be chemical, electrical, or mechanical ii. When the cell is stimulated, the electrical charge inside the cell becomes less negative, and depolarization occurs iii. When the threshold potential is reached, changes occur in the membrane iv. SA and AV nodes achieve threshold potential first v. Cell membrane permeability is altered, and specialized channels in the membrane open, which allows the entry of sodium and calcium ions into the cell g. Action potential: As cardiac cells reverse polarity, the electrical impulse generated during that event creates an energy stimulus that travels across the cell membrane—a high-speed, short-lived, self-reproducing current (heart only). This is represented on an action potential curve (Figure 3-5). i. Phase 0—Depolarization: A quick upstroke (several milliseconds) representing the initial phase of excitation ii. Phase 1—Initial phase of repolarization iii. Phase 2—Plateau phase of repolarization: Slow inward current of calcium (and, to a lesser extent, sodium); potassium diffuses out of the cells iv. Phase 3—Last phase of repolarization: Outward current of potassium increases, and the slow, inward current of sodium and calcium decreases. Cells rapidly repolarize, returning to normal RMP. h. Cardiac pacemaker cells (SA and AV nodes) action potential i. Pacer cells, having increased automaticity, spontaneously depolarize in phase 4 without a stimulus. Other cells of the heart, having repolarized, require another stimulus to become depolarized. (a) Rate of automaticity may be altered by increasing or decreasing the slope of phase 4 (b) Increasing the slope speeds heart rate; decreasing the slope slows heart rate ii. Spontaneous depolarization of pacer cells is caused by a steady influx of sodium and efflux of potassium i. Refractoriness of heart muscle i. Absolute refractory period (effective refractory period): Another stimulus to the cell will not produce another action potential (phases 0, 1, and 2 and part of phase 3 of the action potential curve) ii. Relative refractory period: Only a very strong stimulus can initiate an action potential and cause depolarization (latter part of phase 3) iii. Supernormal period: Weak stimulus (one that would not normally elicit an action potential) can evoke an action potential and cause depolarization (at the end of phase 3) 10. Events in the cardiac cycle (Figure 3-6) a. Ventricular systole: Contraction and emptying of the ventricles i. QRS complex: Represents ventricular depolarization (an electrical event) ii. First phase of ventricular contraction (systole) is called isovolumetric contraction. Pressure increases, but no blood is ejected until LV pressure exceeds aortic pressure (and opens the aortic valve). iii. As pressure rises in the ventricles, the AV valves close, producing the first heart sound (S1, composed of mitral [M1] and tricuspid [T1] components) iv. The “c” wave of the atrial pressure curve is produced when the AV valves are pushed backward toward the atria as ventricular pressure builds v. When LV pressure exceeds the pressure in the aorta, the aortic valve opens (comparable events in the RV occur with the pulmonic valve) vi. Blood is rapidly ejected into the aorta (systolic ejection) vii. LV pressures decrease, falling below the pressure in the aorta, ventricular ejection stops, and the aortic valve closes. (Comparable events occur in the pulmonary artery, closing the pulmonic valve.) viii. Closing of the aortic and pulmonic valves produces the second heart sound (S2, composed of aortic [A2] and pulmonic [P2] components) ix. Aortic valve closure is represented by the dicrotic notch in the aortic pressure waveform x. Repolarization of the ventricles occurs at this time and produces the T wave on the electrocardiogram (ECG) xi. After the aortic valve closes, pressure in the LV falls rapidly (isovolumetric relaxation phase); no blood enters the ventricle xii. LA “v” wave is produced by rapid filling of the atria during ventricular systole, against closed AV valves. This marks the end of systole. b. Ventricular diastole: Filling phase of the ventricles i. When pressure is lower in the ventricles than in the atria, the AV valves reopen, which initiates the early rapid filling phase of the ventricles during diastole. This marks the start of diastole. ii. Pressure in the atria is higher than diastolic pressure in the ventricles, so blood flows from the atria into the ventricles iii. “a” wave: Atrial pressure rises with atrial contraction iv. P wave (ECG): In late diastole, represents atrial depolarization (an electrical event) 11. Variables affecting LV function and cardiac output (CO) a. CO: Amount of blood ejected by the LV in 1 minute i. CO is the product of stroke volume (SV) and heart rate (HR): ii. SV is the amount of blood ejected by the LV with each contraction, or the difference between left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV): SV = LVEDV – LVESV; (60 to 130 ml) iii. Normal resting CO = 4 to 8 L/min iv. CO is determined by preload, afterload, contractility, and heart rate b. Preload: The degree to which muscle fibers are lengthened (stretched) prior to contraction i. In the intact heart, preload is secondary to the volume (size) of the chamber. This is determined by the amount of blood filling the chamber. ii. Increases in preload increase the CO as described by the Frank-Starling law of the heart iii. Muscle fibers can reach a point of stretch beyond which contraction is no longer enhanced, and further increases in preload do not yield any further increase in CO iv. Increased preload occurs with (a) Increased circulating volume (b) Venous constriction (decreases venous pooling and increases venous return to heart) v. Decreased preload occurs with c. Afterload: Initial resistance that must be overcome by the ventricles to develop force and contract, opening the semilunar valves and propelling blood into the systemic and pulmonary circulatory systems (systolic contraction) i. Factors affecting afterload include arterial resistance (wall stress and thickness), aortic impedance, and blood viscosity ii. Systemic vascular resistance (SVR) is used as a rough estimate of afterload iii. To calculate SVR: Mean arterial pressure (MAP) minus central venous pressure (CVP); this number is divided by CO; the resulting value then is multiplied by 80 and converts into dynes/sec/cm−5 (1 dyne is the force that gives a mass of 1 g an acceleration of 1 cm/sec2): iv. Normal SVR = 900 to 1400 dynes/sec/cm−5 v. Excessive afterload: Increases LV stroke work, decreases SV, increases myocardial oxygen demands, and may result in LV failure vi. Increased afterload is seen in vii. Decreased afterload is seen in d. Contractility (inotropic state): Heart’s contractile strength i. There is no way to measure contractility directly. Contractile state can be assessed indirectly through its effects on CO or with noninvasive imaging. ii. Factors increasing the contractile state of the myocardium include (a) Use of positive inotropic drugs (e.g., digitalis, milrinone, epinephrine, dobutamine) (b) Increased heart rate (Bowditch’s phenomenon) iii. Factors decreasing the contractile state of the myocardium include i. Influenced by many factors, including ii. Determinant of myocardial oxygen supply and demand i. CI is CO corrected for differences in body size (CO of 4 L/min may be adequate for a 100-lb woman but inadequate for a 200-lb man) ii. Based on body surface area (BSA) as estimated from a height and weight nomogram: CI = CO/BSA h. Ventricular function curve: Shows how to relate the contributions of preload, afterload, and contractility (but not heart rate) to ventricular function (Figure 3-7) a. Major functions: Provides tissues with blood, nutrients, and hormones and removes metabolic wastes b. Resistance to flow: Depends on diameter of vessels (especially arterioles), viscosity of blood, and elastic recoil in vessel walls c. Circulating blood volume: There is approximately 5 L of total circulating blood volume in the adult body d. Major components of the vascular system (a) Strong, compliant, elastic-walled vessels that branch off the aorta, carry blood away from the heart, and distribute it to capillary beds throughout the body (c) Able to stretch during systole and recoil during diastole because of elastic fibers in the arterial wall (a) Control systemic vascular resistance and thus arterial pressure (b) Have strong smooth muscle walls innervated by the autonomic nervous system (1) Adrenergic (stimulatory) system: Releases two neurotransmitters (epinephrine, norepinephrine). Epinephrine stimulates β-receptors (increases heart rate, increases contractility, dilates arterioles). Norepinephrine stimulates α-receptors (vasoconstriction). (2) Cholinergic (inhibitory) system: Releases acetylcholine (decreases heart rate; releases nitric oxide, causing vasodilatation). (a) Tissue bed exchange of oxygen and carbon dioxide and solutes between blood and tissues; site of fluid volume transfer between plasma and interstitium (b) Gas exchange caused by diffusion. Diffusion of a substance is from an area of high concentration to an area of low concentration until equilibrium is established. (1) Increased capillary hydrostatic pressure moves fluid from the vessel into the interstitium (2) Greater capillary osmotic pressure moves fluid from the interstitium into the vessels (3) Plasma protein concentration in the capillaries provides the osmotic gradient (4) Retains fluid in the intravascular space (5) Prevents edema formation in the interstitium (6) Albumin accounts for 75% of total plasma osmotic pressure; fibrinogen accounts for a small amount (7) Serum albumin level is a good indicator of a patient’s colloid osmotic pressure (a) Stores about 65% of total blood volume (b) Receives blood from capillaries (c) Conducts blood back to the heart within a low-pressure system (d) No muscle layer: Veins are compressed by the contraction of surrounding skeletal muscle (e) Valves in the veins prevent reverse blood flow (f) Venous pressure in the lower extremities is normally 20 mm Hg or less 13. Control of peripheral blood flow a. Autoregulation: Ability of the tissues to control their own blood flow (vasodilatation, vasoconstriction) i. Coronary blood flow remains fairly constant over a wide range of blood pressures ii. As coronary perfusion pressure drops below 50 mm Hg, autoregulatory ability becomes impaired b. Autonomic regulation of vessels i. Vasoconstriction occurs when norepinephrine is released by stimulation of the sympathetic nervous system (adrenergic effect) ii. Vasodilatation occurs when acetylcholine is released by stimulation of the parasympathetic nervous system (cholinergic effect) or by inhibition of vasoconstriction c. Stretch receptors: Baroreceptors (pressoreceptors) keep MAP constant i. Receptor sites in the arteries (aortic arch, carotid sinus, pulmonary arteries, and atria) ii. Action with increased blood pressure (a) Respond to stretching of arterial walls (b) Impulse transmitted from the aortic arch via the vagus nerve to the medulla (c) Parasympathetic nervous system stimulated, sympathetic nervous system inhibited (d) Result: Decreased heart rate and contractility, dilation of peripheral vessels, decreased SVR, decreased blood pressure iii. Action with decreased blood pressure i. Renin-angiotensin-aldosterone system also helps control arterial pressure (see Chapter 5) ii. Renin is a protease secreted by the kidneys; converts angiotensinogen to angiotensin I iii. Renin release from the kidneys is affected as follows: (a) Decreased blood pressure (i.e., hemorrhage, dehydration, diuretics, sodium depletion) → increases in renin secretion (b) Rise in sympathetic output (β stimulation) → increases in renin secretion (c) Fall in sodium concentration → increases in renin secretion (decreased volume) iv. Angiotensin I is converted to angiotensin II. (These effects are blocked by angiotensin-converting enzyme [ACE] inhibitors.) v. Angiotensin II, the most potent vasoconstrictor known, is produced when increased renin secretion stimulates its formation (a) Effects of angiotensin II include the following: (1) Arteriolar constriction, which increases systolic and diastolic pressures (2) Stimulation of the adrenal cortex to secrete aldosterone, which causes sodium and water retention (3) Increase in extracellular fluid volume, which shuts off the stimulus that initiated renin secretion so that blood pressure is maintained at the normal level (b) Effects of angiotensin II are blocked at its receptors by angiotensin II receptor blockers (ARBs) b. Pulse pressure: Difference between systolic and diastolic pressures i. Function of SV and arterial capacitance ii. Normal pulse pressure: 30 to 40 mm Hg iii. Changes in SV (with exercise, shock, heart failure) are reflected in similar changes in pulse pressure c. MAP: Average arterial pressure during the cardiac cycle; dependent on mean arterial blood volume and elasticity of the arterial wall a. Main complaint: Patient’s explanation for seeking medical assistance b. History of present illness: Ascertain the following: ii. Onset: Date, time of day, duration, course, precipitating factors iii. Signs and symptoms: Exacerbations, remissions (a) Discomfort: Character, location, radiation, quality, duration, factors that aggravate or produce, factors that alleviate (b) Fatigue: With or without activity (c) Edema: Location, degree, duration (d) Syncope and presyncope: Onset (presyncopal warning or sudden event), time and circumstances of occurrence (postural, nonpostural, activity), provocative events (cough, micturition, head movement) (e) Dyspnea: Orthopnea, paroxysmal nocturnal dyspnea, dyspnea on exertion (determine how much exercise it takes to elicit in number of blocks, flights of stairs, etc.) (f) Palpitations: Nature, length, associated symptoms (h) Claudication: Hip or calf? How many blocks can you walk? c. Medical history: Identify all previous illnesses, injuries, and surgical procedures i. Patient’s assessment of general health for last several years ii. Risk factors: Hypertension, hypercholesteremia, smoking, family history of cardiac disease, diabetes iii. Last medical examination, hospitalizations, prior relevant cardiac tests (e.g., echocardiography, catheterization) iv. Heart history: Coronary artery disease (CAD), angina, myocardial infarctions (MIs), hypertension, valvular disease, arrhythmias, trauma, peripheral vascular disease, congenital heart defects, heart murmurs, rheumatic fever, cerebrovascular accident (CVA), transient ischemic attacks i. State of health or cause of death and age at death of immediate family members ii. Hereditary, familial diseases pertaining to cardiovascular system i. Present and past work experiences ii. Level of activity, exercise iii. Smoking and drinking habits (present, past) v. Nutrition: Foods eaten, meals per day, who prepares meals vi. Support system: Relationship with significant others f. Medication history: Identify all prescribed or over-the-counter medications. Determine why and how often the patient is taking drug(s), dosages, any side effects, compliance issues. g. Allergies: Medications, foods (i.e., shellfish), environmental substances, iodine (potential reaction to contrast medium used during cardiac catheterization procedures) 2. Nursing examination of patient
The Cardiovascular System
SYSTEMWIDE ELEMENTS
Patient Assessment

The Cardiovascular System
Get Clinical Tree app for offline access