Targeted Therapy
INTRODUCTION
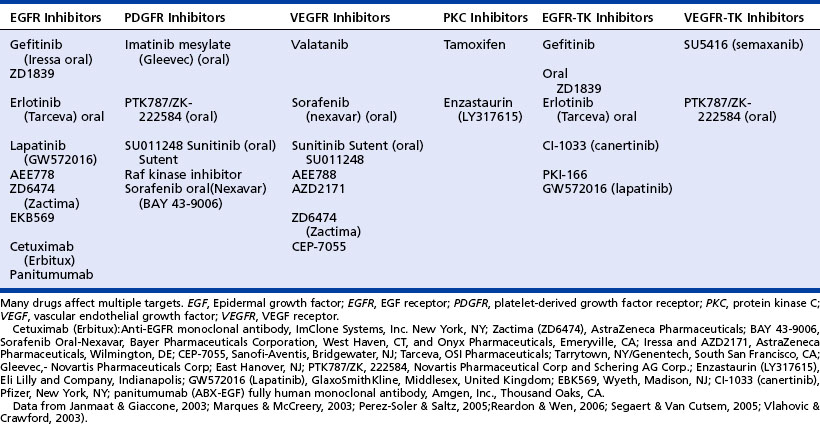
One important issue to keep in mind is that none of the signaling pathways works independently of the other pathways. All cells in the human body contain many different receptors on the cell’s surface, as well as the surrounding microenvironment, which contains many different ligands (i.e., proteins, enzymes) that are set into motion by various steps produced to regulate the normal processes of growth, differentiation, apoptosis, cell adhesion, and angiogenesis. The receptors remain dormant until a specific ligand binds with the receptor, thus initiating a cascade of intracellular signaling pathways, such as the binding of transforming growth factor (TGF)-alpha with the epidermal growth factor receptor (EGFR). A list of terminology specific to the signaling pathways can be found in the box on page 288. The new targeted therapies enhance current treatments, providing the possibility of survival from a disease that has such widespread impact. A list of approved targeted therapies may be found in the table on page 289.
Historical View
For decades, cancers have been treated with various types of treatment modalities. Surgery, the removal of the tumor or abnormal growth, has been the mainstay for most cancer treatment and has been recorded as far back as 3,000 BCE (Niederhuber, 2000). Surgery not only reduces tumor burden as part of the treatment plan but may also be used as prophylaxis, to determine the histologic diagnosis, in determination of disease stage, to relieve pain, and to eliminate or minimize the symptoms of the disease itself.
All these modalities have their own limitations to improving cancer care. Surgery may not remove all the cancer cells. Microscopic disease may still remain, even with the most all-inclusive resections under highly technical navigational systems. Because neither chemotherapy nor radiation therapy differentiates between healthy and cancerous tissues, they cause a variety of nonspecific toxicities sustained by the healthy normal tissues. Poor tolerance of chemotherapy or radiation therapy may result in subtherapeutic dosing or delays in therapy administration schedules or may even necessitate the discontinuation of therapy completely. Furthermore, toxicities to healthy tissues may prevent dose escalation (Herbst & Shin, 2002). In addition, some cancer cells simply may not respond to treatments because of the cellular ability to repair damage or resist drug therapy by expelling chemotherapeutic agents from the tumor cells (Wood & Muehlbauer, 2003). Despite the advances in surgical technology, the refinements in conventional chemotherapy and radiation and development of newer chemotherapeutic agents, many cancers still remain a challenge. Therefore, the new initiative to attack cancer involves treatment that targets specific cellular processes.
MECHANISM OF ACTION
Targeted therapies work differently from chemotherapy in that they interfere with the specific molecules involved in the process of carcinogenesis, tumor growth, and metastasis. The specific molecule being targeted may be the “switch” that regulates growth and development of the tumor cell or the “switch” that allows the cancer cell to enter the process of apoptosis (Wood & Muehlbauer, 2003).
Additionally, targeted therapies have been directed at several of the other cellular changes specific to cancer cells, such as migration of cancer cells or the development of new blood vessels. Because targeted therapies focus on specific molecules or cellular changes, they may be more effective and less harmful to normal cells than other modalities currently available. The benefit of target therapy is a reduction in treatment-related side effects and improvement in quality of life. Targeted therapies are used alone or in combination with other chemotherapeutic agents.
Summary of Targeted Therapies Currently Food and Drug Administration Approved or in Clinical Development
Angiogenesis: the process by which new blood vessels form by sprouting from existing blood vessels
Comedone: a blackhead, discolored dried sebum plugging an excretory duct of the skin
Cytoplasm: the intracellular portion of a cell where biochemical reactions take place
Degradation: the breaking down of a substance
Domain: the functional region or component of a protein
Downstream regulation: changes that take place below the site of a signaling inhibition
Heterodimerization: the pairing of two different (hetero) receptors
Homodimerization: the pairing of two of the same (homo) receptors
Integrins: cell surface proteins that bind to extracellular matrix components
Keratinocytes: cells of the hair, nails, and skin
Malignant: cancerous, a cell/mass that divides and grows without control and order
Monomer: a single receptor in an inactivated state, a molecule of protein
Paronychial: an acute of chronic infection of the folds of skin surrounding the nail
Proteases: enzymes that aid in the breakdown of proteins in the body
Transmembrane: refers to across the cell membrane or through the cell membrane
Tumorigenesis: the change of a normal cell into tumor cells
Tumor suppressor gene: a normal gene that signals the cell to slow down growth and division
Upstream regulation: changes that take place above the site of a signaling inhibition
Data from CancerWeb Project on-line dictionary. (1997-2007). Center for Cancer Education, University of Newcastle-upon-Tyne: http://cancerweb.ncl.ac.uk/cgi-bin/; National Cancer Institute Web site: http://cancer.gov/cancertopics; Esper, P., & Knoop, T. (2005). Current topics in colorectal cancer—targeting VEGF for oncology nurses. Institute for Medical Education & Research: www.imeronline.com, Project ID:04 2605 ES16; McCorkle, M., Gailoto, M., Oestreicher, P., Barkley, D. (2005). Targeted therapies in non-small cell cancer (pp. 1-32). Pittsburgh, PA: Oncology Education Service; Wujcik, D., & Thomas, M. (2005). Current topics in colorectal cancer—targeting EGFR for oncology nurses. Institute for Medical Education & Research: www.imeronline.com, Project ID: 2735 ES 16.
The combination of a molecular targeted therapy and radiation therapy is another promising therapeutic option. Overactivity of the EGFR pathway is associated with radiation resistance. Therefore, combination therapy may increase the effectiveness of radiation (Baumann & Krause, 2004). Because each type of cancer involves a different set of genes and proteins involved in growth and spread, targeted therapies used to control each type of cancer are different. Once tumors can be more accurately classified by molecular and genetic mutations, treatments may then be modified to the individual tumor. The most effective treatment may very well combine the previous modalities of surgery, radiation, and chemotherapy with the newer molecular targeted therapies. The concept of targeted therapies has rapidly expanded just within the last 3 to 5 years and targeted therapies have now become one of the most exciting treatment modalities entering the field of oncology.
ANTIANGIOGENESIS
Angiogenesis is a naturally occurring process in the human body throughout growth and development and specific times during adult life. For the period of embryo development, vasculogenesis is the process that creates the primary network of vascular endothelial cells that become the major vessels (Ferrara, 2004; National Cancer Institute, 2006). Angiogenesis continues throughout fetal development, transforming the new blood vessels and capillaries into a completed circulatory system. From this point and throughout the adult human life the vascular system is generally associated with maintenance controlled by angiogenesis inhibitors. (See the box above for a list of angiogenesis inhibitors.) In the adult, new vessel formation is infrequent and generally associated with the repair of tissue during wound healing and cardiovascular injury (Wood, Sandler, & Muehlbauer, 2005). In women, angiogenesis is also active a few days each month with the formation of new blood vessels in the lining of the uterus during the menstrual cycle.
PROTEINS
TIMP-1 (tissue inhibitor of metalloproteinase-1)
TIMP-2 (tissue inhibitor of metalloproteinase-2)
Data from National Cancer Institute Web site: http://cancer:gove/cancert opics/understandingcancer; Rosen, L.S. (2005). VEGF-targeted therapy: therapeutic potential and recent advances. The Oncologist, 10, 382-391. Available at www.TheOncologist.com.
Angiogenesis is defined as the process by which new blood vessels form by sprouting from existing blood vessels (Wujcik & Thomas, 2005). Tumor angiogenesis is the development of blood vessels that are structurally and functionally abnormal. Both normal physiological and tumor angiogenesis are regulated by a variety of growth factors in their microenvironments. See the boxes on page 289 and right for a designation of inhibitors and activators of angiogenesis. These agents are needed to support the cellular microenvironment of the vascular system.
Other biological functions including extracellular matrix breakdown, proliferation, apoptosis, angiogenesis, and motility are influenced by the reactions created when signals from within the microenvironment influence the ligand binding on the cell surface, creating a signaling cascade inside the cell to the nucleus where the gene transcription is altered and cell functions change (Rempel & Mikkelsen, 2006).
EGFR and vascular endothelial growth factor (VEGF) signaling pathways are two components that play a major role in the process of angiogenesis and the growth and spread of cancer cells. (See the figure on page 291.)
VEGF (a cytokine), as it is commonly called, is also known as VEGF-A. It stimulates vascular endothelial cell growth, survival, and proliferation. It plays a significant role in the development of new blood vessels (angiogenesis). VEGF is a member of a family of six structurally related proteins (see table on page 291) that regulate the growth and differentiation of multiple components of the vascular system, especially blood and lymph vessels. It is also known as for its permeability activity.



