(Advagraf (Apo-Tamox Do not confuse tamoxifen with pentoxifylline, tamsulosin, or temazepam. BLACK BOX ALERT Fatal and non-fatal skin reactions reported. Do not confuse telaprevir with boceprevir or simeprevir. Do not confuse telavancin with dalbavancin or oritavancin; or Vibativ with Vibra-Tabs or vigabatrin.
T
tacrolimus
![]()
![]() , Prograf, Protopic)
, Prograf, Protopic)
tamoxifen
![]()
![]() , Nolvadex-D
, Nolvadex-D ![]() , Soltamox)
, Soltamox)
telaprevir
![]()
telavancin
Nurse Key
Fastest Nurse Insight Engine
Get Clinical Tree app for offline access

 classification
classification classification
classification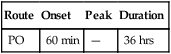
 classification
classification classification
classification classification
classification classification
classification classification
classification classification
classification classification
classification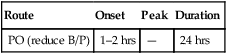
 classification
classification

