18 Support of Renal Function
After reading this chapter, you should be able to:
• summarise the physiology of urine production
• describe the most likely causes of renal failure in the critically ill adult
• differentiate between acute and chronic renal failure
• outline treatment approaches in managing renal failure in critical illness
• appreciate historical developments in dialysis
• describe the indications for renal replacement therapy in critical care
• understand the principles and challenges associated with nursing management of continuous renal replacement therapy in critical care.
Introduction
Sudden deterioration of kidney function, to the point where there is retention of nitrogenous wastes, or acute renal failure (ARF), is a common manifestation of critical illness and is often associated with failure of other organs. Acute renal failure is a syndrome with numerous causes, including glomerulonephritis, prerenal azotaemia, urinary tract obstruction and vasculitis. Acute tubular necrosis (ATN) is a collective term commonly used to describe acutely deteriorating renal function, reflecting pathological changes from various renal insults of a nephrotoxic or ischaemic origin. Factors that cause renal function to deteriorate are not, however, always ischaemic or necrotic in origin, and a syndrome with degrees of failure is often evident. Therefore a new consensus definition and classification system has been established.1 This approach describes staging of ARF severity and embraces the concept of acute kidney injury (AKI) where, like other organs of the body, a dynamic spectrum is found, from small indiscrete changes in function that are immediately reversible, through to gross signs and irreversible organ failure.2
Acute renal failure is defined by a rapid deterioration in renal function (hours to days), which is easily detected by commonly measured markers of kidney performance, including blood urea nitrogen, serum creatinine, and a failed ability to adequately regulate electrolytes, sodium and water balance.3 While generally reversible, ARF can be life-threatening in the critically ill patient if acid–base balance, electrolyte levels (particularly potassium) or fluid overloads are not effectively diagnosed and managed.
The preferred serum marker of renal function is the serum creatinine level. The exact level of serum creatinine that is considered excessive is disputed; however, a doubling of the baseline serum creatinine or levels in excess of 200 µmol/L is commonly agreed on as being indicative of ARF.3 Urine output is also a key factor in determining the severity of ARF. It is well established that oliguric renal failure, that is, a urine output of less than 0.5 mL/kg/h in adults and 1 mL/kg/h in infants, is associated with poorer patient outcome than the non-oliguric form.4
Acute renal failure is reported to occur in 20–25% of intensive care patient admissions, much higher than the broader hospital rate of 5%.5,6,7 In critical care, ARF often forms part of the multiple organ dysfunction syndrome, whose cause has often been associated with sepsis, trauma, pneumonia or cardiovascular dysfunctions (see Chapter 21). Mortality in intensive care ARF is high, with those patients requiring renal replacement therapy (RRT) having worse outcomes than those patients who can be managed without this intervention.8
Related Anatomy and Physiology
Anatomy of Kidneys, Nephron and Urinary Drainage System
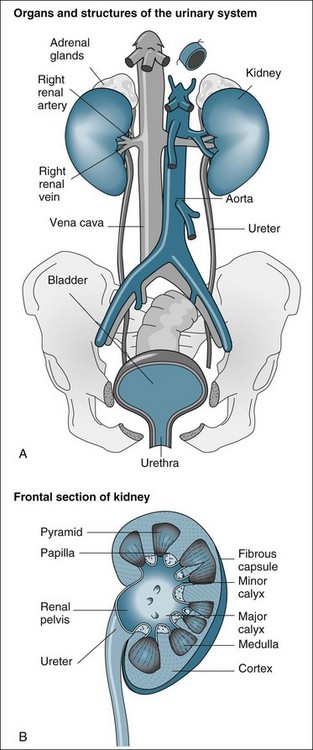
The functional anatomy of the renal system includes the two kidneys, ureters, bladder and urethra (see Figure 18.1). The ureters, bladder and urethra collect, drain and temporarily store the urine produced from each kidney.9 While important in providing the conduit for the final excretion of urine, the kidney is the primary organ of interest in the renal system, particularly in critical care practice, and hence will be described in more detail from the anatomical and physiological perspectives.
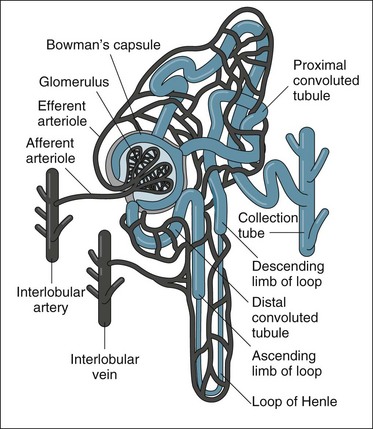
The kidneys are located in the retroperitoneal space on the posterior wall of the abdominal cavity, encased in a protective combination of the ribs, muscle, fat, tendon and the renal capsule. Each adult kidney weighs approximately 140 g. The kidneys may develop a different anatomical appearance, or vary in number and location from the classic description provided here. The functional unit of the kidney is the nephron, which consists of a filtrate-collecting device (the Bowman’s capsule), a convoluted tubule that varies in length and diameter, finally attaching to a common filtrate-collecting tubule and duct (see Figure 18.2). Within the Bowman’s capsule rests the glomerulus, a tuft of interlaced capillaries that arise from the afferent arteriole. The efferent arteriole then drains from the glomerulus via a closely entwined network called the peritubular capillaries, until these collect in the venous network of the kidney.
The glomeruli and nephrons lie in the cortical area of the kidney, while the collecting ducts gather together into the renal pyramids, which lie in the medulla of the kidney. The pyramids drain into the calyces of the kidney, which then drain into the renal pelvis where urine is gathered to drain into the ureter. The major blood vessels of the kidney, the renal artery and veins also enter the renal capsule through the pelvis of the kidney.9
Urine Production, Regulation of Gfr and Filtrate Reabsorption in the Nephron
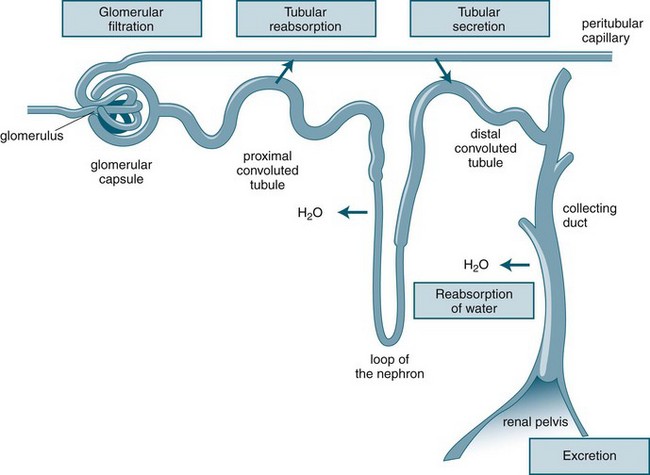
Urine production consists of a three-stage process, which occurs in the nephron: glomerular filtration, tubular reabsorption and tubular secretion (see Figure 18.3). As previously noted, the production of urine is highly dependent on delivery of blood under pressure to the glomerulus. This results in the first step of the urine production process, glomerular filtration. The glomerular filtration rate (GFR) is about 125 mL/min under normal conditions. Changes in the diameter of the afferent and efferent arteriole help regulate glomerular blood flow, but this is unable to compensate for large variations of mean blood pressure; hence, filtration rates may rise or fall markedly over the course of a day.10
As the filtrate transgresses the glomerulus it is collected into the Bowman’s capsule and delivered into the proximal convoluted tubule, loop of Henle and then the distal convoluted tubule, where a number of processes result in the reabsorption of about 99% of the glomerular filtrate. The remaining fluid within the tubule drains into the collecting tubule to form urine. This fluid has substantially different properties from the original glomerular filtrate, as fluid and many electrolytes and glucose are reabsorbed by the peritubular capillaries.10
Along with blood pressure, the sodium content of the extracellular fluid is critical in maintaining fluid balance, as it constitutes the major electrolyte and osmotic agent of the glomerular filtrate. It is imperative that sodium intake and loss is equally balanced, as excessive losses will result in associated fluid loss and excessive intake will result in fluid retention. If sodium balance is not maintained, other compensations such as a rise in blood pressure may result to restore fluid balance. As blood pressure rises, the excretion of sodium also increases by way of the production of additional glomerular filtrate. In this way, water and sodium balance are inextricably linked.10
Hormonal and Neural Regulation of Renal Function
Various feedback mechanisms exist that assist in precisely adjusting the final amount of fluid and electrolyte to be excreted from the kidney. These include the sympathetic nervous system response, angiotension II, aldosterone, antidiuretic hormone and atrial natriuretic peptide. All these mechanisms work in synchrony with blood pressure and sodium balance in ensuring a highly regulated circulating and extracellular fluid volume.10
Renin–Angiotensin–Aldosterone System (RAAS)
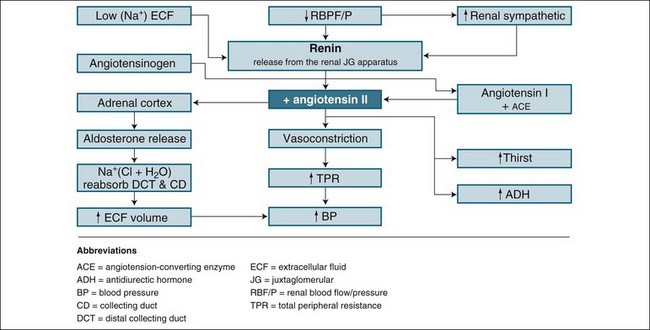
Renin is the chemical trigger to initiate a cascade system that results in two powerful hormones acting on the kidney to significantly influence sodium and water excretion (see Figure 18.4). Renin is produced and released from the juxtaglomerular apparatus, a collection of cells in the macula densa of the distal tubule, and the adjacent afferent arteriole next to the glomerulus, which monitors blood sodium concentration. When released, renin stimulates the activation of angiotensin I from angiotensinogen. Under the influence of coenzyme A, angiotensin I converts to angiotensin II, a potent vasoconstrictor and stimulus to reabsorb sodium and water. The vasoconstrictor effect raises blood pressure and flow to the glomerulus, inhibiting further renin release (a negative feedback mechanism) as perfusion pressure normalises. This allows the return of natriuresis (sodium excretion) and diuresis. This response is essential in assisting with retaining fluid in the event of a falling blood pressure, or boosting fluid excretion as blood pressure rises. It also responds effectively to a rise in sodium intake by reducing angiotensin II formation and allowing a larger natriuresis, resulting in maintenance of sodium balance, a key to tissue fluid distribution and balance.10
Atrial Natriuretic Peptide
Atrial natriuretic peptide (ANP) is a hormone released from the atria of the heart in response to atrial stretching during periods of increased circulating fluid volume. ANP is therefore often described as having an antagonising effect to the RAAS (which acts primarily to preserve sodium and water). These natriuretic, and hence diuretic, effects are mild and self-limiting, and occur in response to mild rises in GFR and reductions in sodium reabsorption. As blood pressure falls, the drop in GFR compensates for the effect of ANP, ensuring that excessive loss of sodium and water does not occur.10
Regulation of Acid–base and Electrolyte Balance
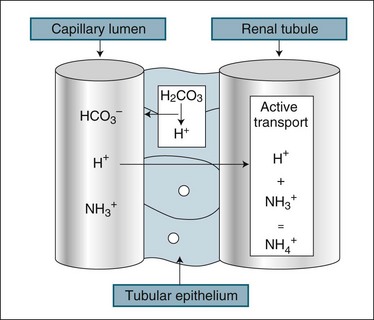
The kidney assists in the management of body pH by regulation of the excretion of H+ and HCO3− ions. While the renal response to alkalosis or acidosis is slow in comparison with plasma buffers and respiratory regulation (see Chapter 13), it does result in a net loss of H+ ions or recovery of HCO3− ions, which are the basis of human pH balance (see Figure 18.5). During acidosis the kidney raises H+ secretion by active transport to combine with ammonia (NH3+) in the renal tubule to form ammonium (NH4+), which is unable to be reabsorbed. Coincidentally, raised H+ excretion increases the reabsorption of sodium, which increases the alkalytic ion, bicarbonate (HCO3−). Conversely, during alkalosis the reabsorption of hydrogen ions is increased. These changes in secretion of hydrogen ion concentration in the renal filtrate alter the pH of the urine down to a maximum level of 4. The buffering of H+ with ammonia reduces the acidifying effect of the hydrogen ions, particularly as some ammonium combines with chloride to form ammonium chloride.10
Role as an Endocrine Organ
The kidney has two homeostatic roles as an endocrine organ. Although neither have effects relevant to acute illness, patients with chronic renal dysfunction often need supplementation to overcome the loss of renal endocrine support. Erythropoietin is important in stimulating the generation of new red blood cells and is released from the kidney in response to a sustained drop in arterial blood oxygen levels. Calcitriol helps regulate the absorption of calcium from the gut, which in turn promotes bone resorption of calcium and the reabsorption of calcium in the kidney. The kidney also acts to convert vitamin D to its active form, which is necessary for the maintenance of body calcium levels.10
Pathophysiology and Classification of Renal Failure
Diseases of the kidneys affect structure and therefore the function of the nephrons in some way. Pathology such as this, if untreated, may not cause complete loss of renal function (i.e. ARF), but is dependent on the amount of nephron damage or ‘injury’ occurring at the time of the illness, and whether the patient has had any previous illness that resulted in undetected kidney damage.11 By focusing on factors that resulted in kidney injury, both individually and collectively, then more serious damage that may result in failure can be averted. This concept is more clearly described in the later section on ATN and AKI which includes the RIFLE Criteria.1,2
The conventional classification of ARF is based on the perceived causative mechanisms, as outlined by numerous authors,12,13 however, irrespective of causative mechanism, the same renal replacement therapies are suitable to treat this:14
Prerenal Causes
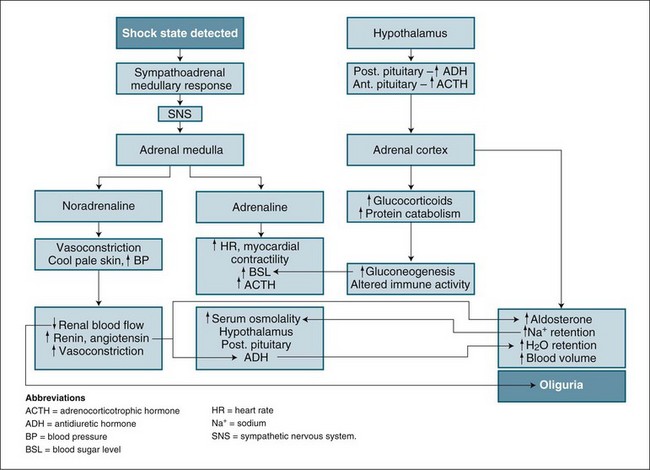
Prerenal factors affecting blood supply to the kidneys, such as hypovolaemia, cardiac failure or hypotension/shock, can cause ARF. The mechanism and outcome are easily related. As blood flow to the kidneys is reduced, less glomerulofiltration occurs, urine production diminishes and wastes accumulate. This state can be reversed by restoration of blood volume or blood pressure. In the short term (1–2 hours), nephrons remain structurally normal and respond by limiting fluid lost by urine production while concentrating the excretion of waste products. The physiological process combines the neuroendocrine control of the hypothalamus and the sympathomimetic response, which then regulates both antidiuretic hormone secretion and the stimulation of the renin–angiotensin–aldosterone system (see Figure 18.4). This process is highly influenced by any preexisting illness or concurrent factors such as diabetes and systemic infection.12
Intrarenal (Intrinsic) Causes
Intrinsic damage to the nephron structure and function can be due to infective or inflammatory illness, toxic drugs, toxic wastes from systemic inflammation in sepsis, vascular obstructive thrombus or emboli. In differentiating this type of ARF, a process of elimination has been suggested where failure of kidney function persists after the restoration of adequate perfusion (blood flow), or where no loss of perfusion has occurred, and there is no obstruction to urine flow.15 Diagnosis is made by exclusion of other causes. The common causes of this type of ARF, glomerulonephritis, nephrotoxicity and chronic vascular insufficiency, are discussed below.
Glomerulonephritis
This condition is caused by either an infective or a non-infective inflammatory process damaging the glomerular membrane or a systemic autoimmune illness attacking the membrane.14 Either cause results in a loss of glomerular membrane integrity, allowing larger blood components such as plasma proteins and white blood cells to cross the glomerular basement membrane. This causes a loss of blood protein, tubular congestion and failure of normal nephron activity. Resolution is based on treating the cause, such as an infection or autoimmune inflammatory illness.16
Nephrotoxicity
Nephrotoxicity occurs as a result of damage to nephron cells from a wide range of agents, including many drugs used in critical care (e.g. antibiotics, anti-inflammatory agents, cancer drugs, radio-opaque dyes).17 Toxic products of muscle breakdown in severe illness and trauma, commonly called Rhabdomyolysis (see Chapter 23 for trauma association),4,13,18 blood product administration reactions and blood cell damage associated with major surgery are also causative agents.19 As these agents may often be given concurrently, a cumulative effect, along with intermittent falls in renal perfusion, may result in the development of intrinsic ARF.
Vascular Insufficiency
One-third of patients who develop ARF in the ICU have chronic renal dysfunction.20 This chronic dysfunction may be undiagnosed prior to the critical illness, and may be related to diabetes, the ageing process and/or long-term hypertension. These factors create a reduction in both large and microvasculature blood flow into and within the kidney, therefore reducing glomerular filtration activity and affecting the reabsorption and diffusive process of the nephron. This reduction in blood flow is exacerbated by degenerative vessel obstruction with atheromatous plaque, particularly pronounced in diabetic patients due to ineffective glucose metabolism. Diabetic patients are more likely to develop ARF associated with medical care in hospital from what may otherwise seem to be a relatively trivial insult to the kidneys in a younger, healthy patient. The event may be enough to trigger ARF in these patients, as they lack any degree of ‘renal reserve’ or tolerance to events such as low blood pressure or administration of nephrotoxic drugs normally filtered by the kidneys.13
Postrenal Causes
Urinary tract obstruction is the primary postrenal cause of ARF, and is uncommon in the critical care setting as it is rarely associated with acute onset renal failure.13 Postrenal obstruction is more common in the community and is associated with urological disorders such as prostate gland enlargement in males, urinary tract tumours and renal calculi formation impairing urine outflow. It is essential that blockage of any urinary drainage device be excluded in the critically ill patient when undertaking an assessment of apparent oliguria.
Acute Tubular Necrosis and Acute Kidney Injury
Intrinsic ARF (described above) is often associated with typical microscopic changes on pathology examination of kidney tissue. This pathology is termed acute tubular necrosis (ATN), and possibly explains how and why, in the acute setting, kidneys can fail abruptly to minimal to no function (no urine output and therefore no waste clearance), and can then after a period of time, with artificial support, recover to normal function in many patients.21 This is an interesting area for current research into the mechanisms responsible for acute kidney failure that are yet to be fully understood.
Acute tubular necrosis describes damage to the tubular portion of the nephron and may range from subtle metabolic changes to total dissolution of cell structure, with tubular cells ‘defoliating’ or detaching from the tubule basement membrane.22 Most ARF is multifactorial in origin and may involve more than one causative mechanism and is not always an ischaemic or necrotic event.15 In critical illness, the most common combination causing ARF is the administration of nephrotoxic agents in association with prolonged hypoperfusion or ischaemia (oxygen deprivation).22 This type of tubular necrosis can be further mediated by infection, blood transfusion reactions, drugs, ingested toxins and poisons, or be a complication of heart failure or major cardiovascular surgery. The initial insult can also be compounded by metabolic disturbances and subsequent systemic infection.
ATN is the causative mechanism for up to 30% of acute kidney failure in the intensive care setting,23 with the precise causative illness not easily identifiable in critically ill patients with multiple co-morbidities, for example diabetes, advanced age, investigations requiring radio-opaque dye administration, potent and nephrotoxic drug administration or major surgery with an inflammatory state due to an underlying infection. This is the context of critical illness and ARF where, despite modulation of the cause and support with artificial renal replacement therapies, mortality ranges from 28–90% depending on diagnostic criteria or definition.3,24,25
This type of kidney damage is of particular importance, as ATN is abrupt in onset and causes a rapid cessation of normal nephron function, a picture typical of any critical illness and failure of other body organs. As this failure is commonly mediated by a loss in total or regional blood flow to the kidney,26 it is more pronounced in the kidney medulla or outer regions sensitive to reduced blood flow. The cause of this loss in blood flow may be multifactorial but is commonly associated with shock and consequent low blood pressure (see Figure 18.6). Tubular cells suffer an ischaemic insult, causing a shedding of the cells from the nephron basement membrane. This shedding of cells has an initial loss of cell polarity, and then cell death, with a ‘patchy’ occurrence along the tubule basement membrane.21 In addition, some cells detach themselves before death in a response known as apoptosis (cell self-death)21 (see Chapter 21). The response is aimed at organ survival, with some individual cells ‘sacrificing’ themselves during a period of crisis. This protective response reduces oxygen demand by initiating cell death in some tubules, while others differentiate and/or proliferate for repair, and allows continuation of some normal function. If the causative process abates, remaining cells regenerate by differentiation and proliferation, tissue repair occurs with restoration of normal epithelium in some tubules and nephron function returns.
During this period cellular ‘debris’ collects in the tubule loops, causing obstruction of tubular flow, with backleak of filtrate occurring through the ‘patchy’ exposed tubular membrane surface. An inflammatory process is also stimulated due to release of cell adhesion factors and leucocyte activation,27 which in turn causes further vasoconstriction and ischaemia28 in the acute stage. The backleakage and static tubular fluid creates a concentrate that, by diffusion, raises blood levels of wastes such as urea, creatinine and other toxins. Along with this cessation of urine flow, toxicity occurs with high serum levels of wastes such as urea, creatinine, potassium and undefined toxins.27 This is the clinical state of ARF associated with the pathology of ATN and now referred to as acute kidney injury (AKI) to better describe the total ‘picture’ of pre-illness status, immediate causative events and degree of injury determined by patient serum creatinine or urine output.1,2
Some authors prefer the term ‘ATN’ to describe ARF,11 using it as a surrogate for ARF in the acute setting, as it focuses on the pathophysiology of tubular damage, recognising this damage as a final outcome of all causative factors. However, more recently, with the development of a consensus definition for ARF describing stages of illness severity, the term acute kidney injury (AKI) is now used reflecting pathophysiology, the outcome of many causative factors, and the clinical context where small derangements may be evident with reversibility of dysfunction and recovery, through to irreversible damage with kidney failure.1,2
The kidneys are vital human body organs essential to sustaining life. An important interrelationship of the kidneys and other body organs exists, with the brain, heart, liver and lungs dependent on receiving ‘clean’ blood to function. As toxins accumulate in ARF these organs become dysfunctional,29,30 although many of these interrelationships (e.g. the liver) are poorly understood.31
Acute Renal Failure: Clinical and Diagnostic Criteria for Classification and Management
Clinical Assessment
Clinical assessment of the patient with impending renal failure can involve myriad tests and investigations; however, the majority of these are not used to assess the critically ill patient. The clinical history is important in differentiating preexisting renal disease and cataloguing the numerous factors already discussed that can contribute to renal dysfunction. As the majority of renal failure patients in the ICU will succumb to the combination of prerenal renal failure and ATN, the key assessments used in monitoring renal function are urine output, serum creatinine and urea levels, combined with more general haemodynamic measures including HR, CVP, BP, PCWP. These measures are essential for the critically ill patient, and alterations provide the diagnostic key. They also link into the wider assessment of fluid and electrolyte balance, as described in Chapters 9 and 19.
Diagnosis
The management of ARF begins with making the diagnosis, based on the patient’s presenting signs and symptoms linked to a patient history. A long-term history of renal disease involving urinary tract infections, diabetes, cardiac failure and systemic inflammatory illnesses are all highly relevant.11 Immediate history of presentation to a hospital involving surgery or any life-threatening illness with associated shock is also highly relevant in association with reduced urine output volumes over time.
Nurses in the critical care setting who measure urine output hourly, readily recognise a key sign of impending renal dysfunction. Oliguria in the absence of catheter obstruction should be responded to quickly, as this suggests inadequate kidney blood flow and to some extent is a delayed observation considering the continual moderation of kidney function producing urine output. Persisting oliguria or the onset of anuria with associated rises in blood creatinine defines renal failure. This sequence of events can be identified in a criteria pathway published following a consensus meeting of physicians. The ‘RIFLE’ criteria32 – risk, injury, failure, and outcome criteria of loss and end-stage disease – provides an increasingly widely-accepted approach to diagnosing and classifying ARF.
Consensus Definition: the Rifle Criteria
The RIFLE criteria are indicated in Figure 18.7, and use raising creatinine and lowering urine output as highly sensitive and specific indicators for a continuum of renal failure. This is a useful classification system to grade loss of kidney function, reflecting stages of injury to the kidney before failure occurs. Without this, the small but important losses of kidney function before the failure state are not adequately considered.1 This approach provides a consensus definition for loss of kidney function that is useful for clinical practice and research into this area, with clinicians all talking the same ‘language’ when comparing patients and/or results from clinical trials. The shape of the diagram indicates that more people will develop symptoms of ARF linked to kidney ‘injury’ and be considered ‘at risk’ (high sensitivity) than those at the bottom of the definition, who are fewer in number but need to fulfil strict criteria (high specificity).
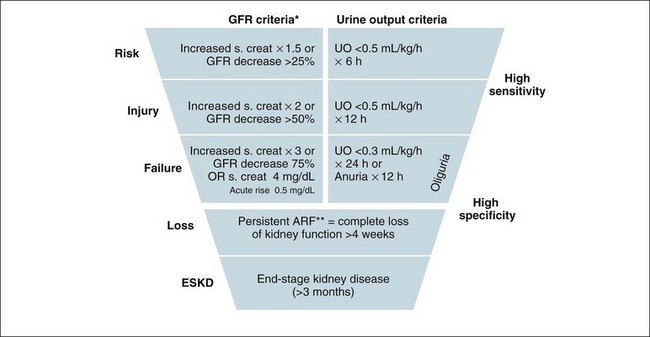
FIGURE 18.7 Criteria for diagnosis of acute renal failure: the risk, injury and failure criteria with outcomes of loss and end-stage renal disease (RIFLE).32
To better understand the RIFLE criteria in a clinical care context, the following discussion is useful to consider in association with a review of Figure 18.7. In a hospital setting, those patients with a risk of renal injury would be identified by a low urine output of less than 0.5 mL/kg/h for 6 hours. In this situation their creatinine would be expected to rise, indicating a concurrent reduction in glomerular filtration rate. Reducing renal risk requires basic measures such as increasing their fluid intake and continuing to closely monitor urine output, whilst reviewing the patient’s medications, haemodynamic state and possible other causes of injury in order to prevent further deterioration.
Clinical Management
In the critically ill patient, kidney function failure may be associated with an initial renal response to a fall in perfusion associated with systemic shock. As the majority of patients recover their renal function from ICU ARF,8 initial clinical management is aimed at reducing further renal damage. If kidney function becomes so compromised that blood pH, fluid and electrolyte balance cannot be sustained, then a replacement therapy will need to be introduced. This is continued until kidney function is marked by the return of urine production or patients are moved to a more chronic form of replacement therapy, such as intermittent haemodialysis.
Reducing Further Insults to the Kidneys
After diagnosis, the next management principle is to remove or modify any cause that may exacerbate the pathological process associated with ARF. Further interventions and investigations are performed in relation to the findings from the history and presentation. These may include:13
• further intravenous fluid resuscitation (despite an oligo-anuric state) and restoration of blood pressure
• physical or diagnostic assessment for renal outflow obstruction and alleviation if present
• ceasing or modifying the dose of any nephrotoxic drugs or agents and treating infection with alternative, less toxic antibiotics.
Initial management strategies for developing ARF remain conservative, with careful management of fluid (once adequate circulating volume is assured by initial fluid resuscitation) and haemodynamics, encouraging diuresis if present, monitoring blood profiles for changes in urea and electrolytes, and limiting or reformulating the administration of agents that may contribute to the accumulation of urea and electrolytes (e.g. enteral or IV nutritional supplements). The use of agents such as mannitol, dopamine and frusemide, while popular in practice, have not been shown to be of any value in improving outcome in patients at risk of ARF.13,20
Despite these efforts, life-threatening biochemistry may arise in ARF, such as severe acidosis and hyperkalaemia (a pH of <7.1 and a serum potassium >6.5 mmol/L) that requires immediate treatment and can be an indication for beginning RRT without elevation of serum creatinine and oliguria and fluid overload.33
Nutrition
When ARF is persistent, providing nutritional support is another important management strategy. A review of nutrition in ARF suggested that an intake of 30–35 kcal/kg/day and a protein intake of 1–2 g/kg/day is essential due to the combined increase in protein catabolism and caloric requirements of associated critical illness.34 This nutrition is provided by the enteral or parenteral route and constitutes an increase in body fluids and nitrogen load. Ironically, this aspect of managing ARF in critical illness may also be an indication for starting RRT35 (see Chapter 19 for further discussion on nutritional requirements in critical illness).
Renal Replacement Therapy
If conservative measures fail, then the ongoing management of the patient with ARF requires RRT. This enables control of blood biochemistry, prevents toxin accumulation, and allows removal of fluids so that adequate nutrition can be achieved. The criteria and indications for initiating RRT are listed in Box 18.1. One indication is sufficient to initiate RRT, while two or more make RRT urgent and mandatory. Combined derangements can create the necessity to commence therapy before individually-defined limits have been reached. Early initiation of treatment is widely advocated and may confer more rapid renal recovery.
Box 18.1
Proposed criteria for the initiation of renal replacement therapy in adult critically ill patients14
Renal Dialysis
History
Dialysis is a term describing RRT and refers to the purification of blood through a membrane by diffusion of waste substances.36 Table 18.1 outlines the historical events in the development of dialysis. The Kolff rotating drum kidney, one of the earliest attempts at RRT (illustrated in Figure 18.8), used cellulose tubing rolled around a wooden skeleton built as a large, drum-styled cage. Cellulose acetate (material similar to ‘sticky tape’) tubing was strong, did not burst under pressure and could be sterilised.37 The drum with the blood-filled cellulose tubing wound around it was immersed in a bath of weak salt solution, and as blood passed through, the rotating cellulose tubing allowed waste exchange to occur by diffusion. This method and the diagram included is useful information as it is essentially the key concepts for how dialysis is today with modern machines and dialysis membranes. A major impediment to the safe use of this system was, however, the large amount of patient blood required in the tubing and membrane.
TABLE 18.1 Historical events in the development of dialysis
| Time period and developer | Description |
|---|---|
| 1854: Thomas Graham, Scottish chemist | First used the term ‘dialysis’ to describe the transport of solutes through an ox bladder, which drew attention to the concept of a membrane for solute removal from fluid. |
| 1920s: George Haas, German physician | First human dialysis was carried out, performing six treatments on six patients. Haas failed to make further progress with the treatment but is recognised as an early pioneer of dialysis. |
| 1920–30s | Synthetic polymer chemistry allowed development of cellulose acetate, a membrane integral to the further development of dialysis treatments. |
| 1940s: Willem Kolff, Dutch physician | The discovery of heparin, an anticoagulant, enabled further development of dialysis during World War II, the Kolff rotating drum kidney. |
| 1940–50s: Kolff and Allis-Chalmers, USA | Further modification of Kolff dialyser and the development of improved machines. |
| 1950s: Fredrik Kiil, Norway | Developed the parallel plate dialyser made of a new cellulose, Cuprophane. This required a pump to push the blood through the membrane and return the blood to the patient. |
| 1950–60s | Dialysis began to be widely used to treat kidney failure. |
| 1960s: Richard Stewart and Dow Chemical, USA | The hollow-fibre membrane dialyser used a membrane design of a cellulose acetate bundle, with 11,000 fibres providing a surface area of 1 m2. |
| 1970s | Use of first CAVH circuits for diuretic resistant oedema by Kramer |
| 1980s | First continuous therapies using blood pump and IV pumps to control fluids removal and substitution: Australia and New Zealand led the way |
| 1990s | New purpose built machines used; Gambro Prisma, Baxter BM 11 + 14 to provide pump controlled therapies with integrated automated fluid balance using scales to measure fluids. Cassette circuits, automated priming; new membranes |
| 2000 | Further purpose built machines using direct measurement for waste and substitution fluids via Hygieia–Kimal machine. Introduction of high fluid exchange rates for sepsis treatment. introduction of dialysis based machines in ICU for daily ‘hybrid’ treatments: SLEDD and SLEDDf |
| 2010 | Multiple CRRT machines; more advanced graphics interface and smart alarms. Waste disposal systems. High flux, porous membranes |
This large extracorporeal blood volume became a focus for further development of the therapy. The goal was to develop a membrane for solute exchange with a greater surface area than the cellulose membrane used by Kolff but needing less blood volume. This led to the development of the hollow-fibre filter membrane structure in the 1960s, the same design concept that is used today. Since then significant developments have occurred, with new fibres using the polymer polysulfone or other artificial synthetic chemical structures that better imitate the nephron glomerulus and the ability to transfer wastes and plasma water38 for an effective ‘artificial kidney’.
This combination of extracorporeal circuit (EC), blood pump and filter membrane (or artificial kidney or dialyser), and the associated nursing management is now commonly known as haemodialysis. The major treatment components are essentially the same as those first developed in the 1960s, with the key component being the device membrane. Over the past 50 years, industrial and scientific developments such as plastics moulding and electronics have made current dialysis techniques safe, effective and a life-sustaining treatment for the millions of people who suffer acute and chronic kidney failure.39,40
Historical Perspectives of Dialysis Nursing
Key to the application of these technical and scientific developments has been the role of nurses, who have made a substantial contribution to the safety and efficiency of dialysis. Barbara Coleman is recognised as the first dialysis nurse to publish a treatment protocol for dialysis using the rotating drum machine in 1952.39,41 Nursing of dialysis patients has developed into a specialist field of knowledge and skill, with nurses combining their holistic view of patient management with the specialist needs of patients with renal failure, from the outpatient setting to the ICU,42 including a collaborative approach to further adaptations of dialysis best suited to the critically ill.43,44
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree