The young person with type 1 diabetes mellitus
CASE AIMS
After examining this case study the reader should be able to:
• Briefly explain what is meant by type 1 diabetes.
• Discuss the possible causes of type 1 diabetes.
• Explain why type 1 diabetes can lead to ketoacidosis and coma.
• Discuss the normal formation and function of insulin.
• Describe why insulin is prescribed to people with type 1 diabetes.
• Outline why a young person’s blood glucose may be difficult to stabilize.
• Discuss the role of the nurse in assisting a patient with type 1 diabetes and their family to maintain their insulin regimen.
CASE
Jamie is a 15-year-old who was diagnosed with type 1 diabetes mellitus at the age of 3 when he was admitted to hospital with diabetic ketoacidosis in a comatose state. At initial diagnosis Jamie’s blood pH was 7.18 and his blood glucose (BG) level was 7mmol/L. Although he had shown signs of weight loss and excessive thirst, at the time his parents thought he was ‘coming down with something’. They were devastated when they were told that their son had diabetes and were not fully prepared for the impact this would have on their lives.
1 What is diabetes mellitus?
2 Discuss the possible cause of Jamie’s type 1 diabetes
3 Explain why Jamie’s diabetes led to ketoacidosis and coma
4 Discuss the normal formation and function of insulin
5 Why has Jamie been prescribed insulin?
Over the past six months Jamie’s parents have been extremely anxious as he has been experiencing severe nocturnal hypoglycaemic attacks associated with seizures. His mother realizes that he has been drinking alcohol when out with friends and tries to get him to eat toast or cereal when he comes in; however, she is not always around. She is also concerned as Jamie has been eating out but missing his insulin in an attempt to appear ‘normal like his mates’.
6 Why may Jamie’s blood glucose be difficult to stabilize at his age?
7 Identify the role of the nurse in assisting Jamie and his family to maintain his insulin regimen
ANSWERS
1 what is diabetes mellitus?
A Type 1 diabetes is a complex and chronic long-term condition in which the child or young person cannot metabolize carbohydrate or sugar due to a lack of insulin production by the β-cells of the Islets of Langerhans in the pancreas and the resultant disturbance of protein and fat metabolism (Hirschorn 2003; McCance and Huether 2010). The incidence of diabetes in childhood has increased over the last 20 years and according to Diabetes UK (2010) now affects almost 3 children per 1000 by the age of 18 years in england, a figure reflected in other parts of the UK (National Centre for Social Research 2008; NHS Scotland 2009). The condition is rare before 9 months old and peaks at 12 years of age (DH 2010).
2 Discuss the possible cause of Jamie’s type 1 diabetes
A The Interplay of both environmental and genetic factors is Implicated in causation, although most authors concur that there is no one, known, cause (King 2003; Meetoo et al. 2007).
GENETIC INHERITANCE
According to the Scottish Intercollegiate Guidelines Network (SIGN 2010), 12–15% of young people under 15 with diabetes have an affected first-degree relative. SIGN also points out that children are three times more likely to develop diabetes if their father has or had the disease than if their mother has or had it. Of patients with CF, 20% will develop secondary diabetes by the age of 20, with the incidence increasing to 80% by 35 years. Genetic factors appear to play a greater part in children who are diagnosed with diabetes under the age of 5, than at any other time (Raine et al. 2011).
Maturity onset diabetes of the young
Maturity onset diabetes of the young (MODY) is the most recently identified cause of diabetes in young people. MODY is a familial autosomal dominant condition that affects children of affected parents. All children of an affected parent with MODY have a 50% chance of inheriting the affected gene and developing MODY themselves (Matyka et al. 1998; Kirby 2005).
Autoimmune theory
Heredity appears to be mainly via the human leukocyte antigen (HLA) complex, which is determined by chromosome 6 in humans, resulting in an intolerance of IA-–2 (insulinoma-associated tyrosine phosphatase-like protein, or islet cell antigen 512 [ICA512]). This leads to destruction of the β-cells with small islets and subsequent decline in insulin production. There is frequent presence of macrophages, T and B lymphocytes and natural killer cells. However, there is evidence that less than 10% of those children and young people with HLA-conferred diabetes susceptibility do progress to develop the clinical disease, again highlighting the multifactorial nature of diabetes (Knip et al. 2005; Daneman 2006; Marieb et al. 2010).
ENVIRONMENTAL FACTORS
A number of theories have been postulated regarding the aetiology of type 1 diabetes. The most important environmental factors appear to be infectious, dietary, perinatal and psychosocial. Enteroviruses (especially Coxsackie B virus), breastfeeding, the early presence or lack of certain foods, birth weight, childhood over-nutrition, maternal islet autoimmunity and negative stress events have been shown to be related to the prevalence of type 1 diabetes. There is also an increased risk if the child has autoimmune co-morbidity such as rheumatoid arthritis, hypothyroidism, Addison’s disease or celiac disease, or if there is a family history (Davendra et al. 2004; Porth 2005; Peng and Hagopian 2006).
3 Explain why Jamie’s diabetes led to ketoacidosis and coma
A
• In Jamie’s circumstances the β-cells of the pancreas have been destroyed and so insulin is not produced, causing the blood glucose level to rise above the normal values of 4–5.9mmol/L (SIGN 2010).
• In an attempt to regain homeostasis, a hyperosmolar diuresis occurs where glucose, sodium and potassium are excreted in the urine, leading to increased fluid loss, hyponatraemia, hypokalaemia and dehydration, which is when Jamie would become thirsty (DH 2010).
• This is enhanced by dehydration as the acids cannot be secreted into the urine for excretion and so the cycle escalates.
• In an attempt to rid the body of the excessive acids in the form of carbon dioxide and carbonic acid, the respiratory rate increases and deepens (Kussmaul’s respirations), and breath may be noted to smell of ‘pear drops’ or ‘plaster remover’, but this is not universal.
• The protein carrier GLUT (glucose transporters) 3 cannot convey glucose into the neurons at acidotic pH levels, and so Jamie would experience increasing confusion and eventual loss of consciousness.
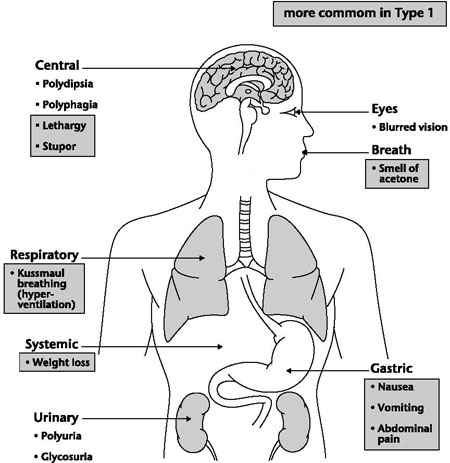
Figure 9.1 Main symptoms of diabetes
4 Discuss the normal formation and function of insulin
A NATURAL INSULIN FORMATION
Insulin is composed of a complex form of amino acid polypeptide which is synthesized in the β-cells as a monomer (single insulin molecule unit) and is stored in a readily available inactive hexamer (6 insulin molecule unit) granule form in the pancreas.
Approximately one to two hours following a meal, insulin is secreted every three to six minutes from the pancreas in pulses into the portal vein, which brings variable amounts of blood into the liver depending on the ingestion of food (Nair 2007a; White and Porterfield 2007). This pulsatile timing is thought to assist the liver in extracting insulin from the blood while maintaining an even blood glucose supply to the cells.
ACTION OF INSULIN
The only mechanism by which cells can take in glucose is via facilitated diffusion (Nair 2007b). A specialized group of proteins known as facilitated GLUT 1–4 transport glucose into specific cell types, across the plasma cell membrane (White and Porterfield 2007). These are:
• GLUT 1, which is responsible for the uptake of glucose and vitamin C by erythrocytes and barrier membranes such as the blood–brain and blood–testicular barriers. The entrance of glucose into erythrocytes generates glycosolated haemoglobin (also known as HbA1c) that is used as the determinant for the stability of diabetes (normal range 4–5.9%).
• GLUT 2, which also carries glucosamine and enables the pancreas and liver to utilize glucose and facilitates renal reabsorption of glucose.
• GLUT 3 is the most important carrier of glucose for sperm, white cells and neurons as it has the highest affinity for glucose and a fivefold greater transport capacity than any of the other transport proteins.
• GLUT 4 is the only insulin-dependent carrier protein and is found in myocytes in striated muscle and adipocytes in fat, which are the main reactor cells to insulin. Where insulin is low, GLUT 4 is locked in an inactive state into intracellular vesicles of fat and muscle. Once in contact with insulin, a protein cascade system is implemented that results in the vesicles being translocated to the cell membrane with which they fuse. GLUT 4 transporters are inserted and become available for transporting glucose across the cell membrane into the cell, resulting in increased glucose absorption (Butler and Kirk 2011).
In order to provide the most efficient energy source for the body, the process of glycolysis is employed. This is the way in which a single molecule of glucose (a 6 carbon sugar) is reconstituted into two three-carbon sugars while generating two molecules of adenosine triphosphate (ATP), two molecules of reduced nicotinamide adenine dinucleotide (NADH), two of pyruvic acid and two of water. This is a process that can occur with or without the presence of oxygen; however, if oxygen is present the process is much more energy efficient (Xuxia and Garvey 2010).
The actions of insulin (indirect and direct) on cells include (Lewis 2000; Kirby 2005; Daneman 2006; Nair 2007a; Holt et al. 2010; Porth 2010; Butler and Kirk 2011):
• Increased glycogen synthesis – insulin facilitates the storage of glucose in liver and muscle cells in the form of glycogen. Lowered levels of insulin cause liver cells to convert glycogen to glucose and excrete it into the blood.
• Decreased gluconeogenesis – decreases production of glucose from non-sugar substrates, primarily in the liver, as the majority of endogenous insulin arriving at the liver is utilized here. Lack of insulin causes glucose production from assorted substrates in the liver and elsewhere.
• Increased lipid synthesis – insulin enables fat cells to take in blood lipids, which are converted to triglycerides; lack of insulin causes the reverse.
• Decreased lipolysis – insulin creates a reduction in the conversion of fat cell lipid stores into blood fatty acids. A lack of insulin reverses this process.
• Increased esterification of fatty acids – adipose tissue manufactures triglycerides from fatty acid esters. A lack of insulin causes the reverse.
• Decreased proteolysis – decreases the breakdown of protein.
• Increased amino acid uptake – where circulating amino acids are absorbed by the cells, while a lack of insulin inhibits absorption.
• Increased potassium uptake – via the absorption of serum potassium. Lack of insulin inhibits absorption.
• Arterial muscle tone – causes arterial wall muscle to relax, increasing blood flow, especially in microarteries. Lack of insulin reduces flow by allowing these muscles to contract.
• Increased secretion of hydrochloric acid – by parietal cells in the stomach.
• Decreased – renal sodium excretion.
DEGRADATION OF INSULIN
Once the insulin molecule has fused with the receptor in the cell plasma wall and effected its action, it may be released back into the extracellular environment or be degraded by the cell. This is mainly effected in the liver and the kidneys. While the liver clears most insulin during first-pass transit, the kidneys do so in the systemic circulation. Degradation normally involves endocytosis of the insulin-receptor complex, where it is engulfed by the cells, followed by exposure to insulin-degrading enzyme (IDE) which breaks the polypeptide B bond of insulin. An insulin molecule produced endogenously by the pancreatic β-cells is estimated to be degraded within about an hour of its initial release into circulation (Levy 2011).
5 Why has Jamie been prescribed insulin?
A Type 1 diabetes is controlled by giving insulin. As we have discussed this helps glucose to be absorbed into the cells and converted into energy, which stops it building up in the person’s blood. Insulin injections are the most common form of treatment, where the patient injects insulin under their skin. Jamie will usually inject himself before meals, using either a small needle or a pen-type syringe with replaceable cartridges. There are several different kinds of insulin that work at different rates and act for different lengths of time (see Table 9.1).
| Insulin type and action | |
| Rapid acting analogue (clear) | |
| Onset: | 10–15 minutes |
| Peak: | 60–90 minutes |
| Duration: | 4–5 hours |
| Fast acting (clear) | |
| Onset: | 0.5–1 hour |
| Peak: | 2–4 hours |
| Duration: | 5–8 hours |
| Intermediate acting (cloudy) | |
| Onset: | 1–3 hours |
| Peak: | 5–8 hours |
| Duration: | up to 18 hours |
| Long acting (cloudy) | |
| Onset: | 3–4 hours |
| Peak: | 8–15 hours |
| Duration: | 22–26 hours |
| Extended long-acting analogue | |
| Onset: | 90 minutes |
| Duration: | 24 hours |
| Premixed (cloudy) | |
| These insulins are presented as a single vial which contains a fixed ratio of insulins (a percentage of rapid/fast-acting to a percentage of intermediate/long-acting) | |
6 Why may Jamie’s blood glucose be difficult to stabilize at his age?
A
• Vacillations in blood glucose levels during adolescence are relatively common. Hyperglycaemia (any blood glucose above 10mmol/L) can be due to the increase in growth hormone secretion associated with a rapid increase in size, which is frequently accompanied by increased appetite. This may call for an increase in insulin dose up to 2 units/kg/day), which the adolescent may not administer in an attempt to prevent hypoglycaemic attacks (SIGN 2010; Wilson 2010; Raine et al. 2011).
• Hypoglycaemia (blood glucose below 4mmol/L), is most common at this period in life as many young people undertake exercise or dance as part of a healthy lifestyle, which rapidly uses up glucose and hence their insulin supply. In an attempt to avert this, young people prefer to run their ‘sugars high’ (Wilson 2008, 2010a; Raine et al. 2011).
• Wilson’s research in 2010 highlights the dilemmas that young people can experience when attempting to socialize and fit in with their social peers: ‘I find the pressure to drink in order to have a good time difficult when my mates all want to go to the pub at weekends and, even if I have orange juice, it still puts my blood [glucose] up. Once, someone put vodka in my orange juice and my blood sugar shot down, then up again really high later on. It was a nightmare. You can’t give yourself more insulin as you don’t know what’s working from your evening meal. Eating out and injecting at different times is also difficult. It’s easier to just enjoy yourself and not worry about the diabetes’ (p. 27).
7 Identify the role of the nurse in assisting Jamie and his family to maintain his insulin regimen
A
• The role of the nurse is to perform an exacting assessment of the young person’s lifestyle that includes the family. The stress and anxiety that family members can suffer with regard to the child with diabetes has been identified, while young people felt that they matured faster than their peers but that taking responsibility for self could be difficult because of this parental protection and anxiety (Grey 2000; Grey et al. 2000; Huss and Enskar 2007; Streisand et al. 2010).
• Health promotion with regard to healthy eating, exercise, the use and abuse of insulin, injection site maintenance, effects of alcohol consumption on blood glucose levels and its impact in terms of longer-term complications should be undertaken (NICE 2003; Nair 2007b; Butler and Kirk 2011).
• Monitoring blood glucose levels is an important part in the nursing management of children and young people with diabetes. It is recommended (Diabetes UK 2010) that patients with diabetes should aim to maintain their blood glucose levels at 4–6mmol/L before meals (preprandial) and 9mmol/L or less two hours after meals (postprandial). When children are young their blood glucose should be above 10mmol/L prior to bed while older children should have a blood glucose of above 7mmol/L to prevent nocturnal hypoglycaemia (NICE 2004).
• HbA1c should be monitored annually to assess the patient’s reporting veracity. This is especially the case with young people, who, like Jamie, may find controlling diet and balancing lifestyle with insulin very difficult.
• If, as with Jamie, hypoglycaemia does occur, then a short-acting carbohydrate such as glucose tablets or drinks, or a snack-sized chocolate bar should be eaten followed by a longer-acting and more complex foodstuff such as bread, cereal or pasta to prevent a reoccurrence.
• Glucose gel rubbed on the inside of the mouth may be necessary if the child or young person is unable or unwilling to eat. If all else fails, intramuscular glucagon (0.5mg if body weight is less than 25kg or 1mg if more than 25kg) (SIGN 2010; Raine et al. 2011).
KEY POINTS
• Diabetes mellitus is one of the most common chronic conditions of childhood.
• The causes remain vague, but genetic, environmental and social factors have been implicated.
• The role of the nurse is assisting the individual and family to maintain stable blood glucose control in an effort to delay the onset of complications.
• Young people should be informed of the importance of maintaining insulin and carbohydrate balance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


