Chapter 36 STDs and syphilis
Introduction
As sexual transmission is the primary mode for the spread of HIV infection, individuals at risk for STDs are also at risk for HIV infection. Approximately one in five HIV-infected individuals worldwide is co-infected with a second sexually transmitted infection [1]. Additionally, the presence of untreated STDs has been shown to increase the acquisition and transmission of HIV. Therefore, all individuals infected with STDs should be treated and tested for HIV infection. Furthermore, recognition of a new STD in an HIV-infected patient is an indication of unprotected sex with the potential for HIV transmission in the community.
Syphilis
Epidemiology
About 90% of syphilis cases worldwide are in low-income countries, with sub-Saharan Africa and South and Southeast Asia carrying most of the burden (9.6 and 9.2 million cases, respectively, in 1999) [2]. Every year over 2 million pregnant women are diagnosed with syphilis [3]. In North America, Western Europe, and Australia, a majority of new syphilis cases are among men, particularly men who have sex with men [4, 5].
Because syphilis may facilitate HIV acquisition and transmission, and because syphilis and HIV have similar modes of transmission, co-infection is common. In the United States approximately 10-20% of syphilis patients are co-infected with HIV; in Rwanda the prevalence of syphilis in HIV-infected women was almost twice as high as in HIV-uninfected women [6]. All patients with syphilis should therefore be tested for HIV infection, and HIV-infected patients should be screened at their initial visit and regularly thereafter with the frequency dependent on risk behavior.
Natural history, pathogenesis, and pathology
Syphilis is caused by infection with Treponema pallidum pallidum. The primary mode of transmission is through sexual contact. Syphilis is readily transmissible by oral sex and through vaginal and anal intercourse. Syphilis can also be transmitted vertically (in utero and at delivery) and less frequently by blood transfusion [5].
Syphilis occurs in three distinct stages: primary, secondary, and tertiary. Once infected, there is an approximately three-week incubation period before the initial lesion of primary syphilis develops. Primary syphilis lasts from a few days to several months and may overlap with the signs and symptoms of secondary syphilis in about one-third of patients (this increases to up to three-fourths among HIV co-infected patients.) Secondary syphilis presents between 1 to 3 months after infection and is followed by latent syphilis. Without therapeutic cure, approximately a third of patients will progress to tertiary syphilis [7].
Because syphilis is readily treatable with antibiotics, the natural history has not been well-studied. However, the Oslo syphilis natural history study conducted from 1891 to 1951 found a mortality rate of 15% for men and 8% for women [8].
Clinical features
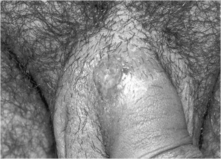
Syphilis is a systemic disease that has symptomatic periods that alternate with periods of clinical latency. Primary syphilis is characterized by an ulcer or chancre at the site of inoculation (see Fig. 36.1). Usually the lesion is solitary, but HIV co-infected patients are more likely to have several lesions that may be larger and deeper than those in HIV-uninfected patients. Approximately 80% of patients also develop unilateral lymphadenopathy near the site of the lesion [5, 7].
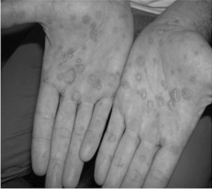
Secondary syphilis typically presents as a diffuse non-pruritic rash consisting of many 3- to 10-mm pink, red, or copper-colored flat lesions or macules. The rash most often affects the trunk and limbs, and raised circular lesions (papulosquamous) with fine circumferential scaling are found on the palms and soles in some cases (see Fig. 36.2). In addition to the rash, 5–10% of patients may experience patchy hair loss. Some patients may experience diffuse lymphadenopathy, pharyngitis, or fever [5, 7].
Although the symptoms of secondary syphilis will abate even in the absence of treatment, up to 25% of patients will experience a recurrence of secondary syphilis, usually within the first year. The period during which no symptoms are present is termed latent syphilis, and although sexual transmission during latency is unlikely, the possibility of mother-to-child transmission remains [7].
Up to a third of patients with untreated syphilis will develop tertiary syphilis. Characterized by a broad range of long-term complications, tertiary syphilisis generally divided into three sub categories: cardiovascular syphilis, gummatous (late benign) syphilis, and late neurosyphilis. Cardiovascular syphilis is the most common manifestation, although it is on the decline, probably as a result of the widespread use of antibiotics. The most common complication is disease of the aorta resulting in aortic regurgitation or aortic aneurysm, usually of the ascending arch. Gummatous syphilis is named for the granulomatous-like lesion most commonly found in the skin, bone, and liver. Although rarely physically debilitating, those lesions can present in any organ and can therefore cause complications. Incidence peaks approximately 15 years post infection [5, 7, 9].
Neurosyphilis is caused by invasion of the cerebrospinal fluid (CSF) by T. pallidum and can occur at any stage of disease. Most patients who experience early treponemal invasion of CSF will have spontaneous resolution. For others, early neurosyphilis is characterized by meningovascular disease, acute and subacute myelopathy, brainstem or cranial nerve abnormalities, or vestibular and ocular abnormalities [10]. Partial loss of movement and muscle weakness and abnormal physical sensations are classic late-stage neurosyphilis syndromes as well as dementia [5, 7].
Patient evaluation, diagnosis, and differential diagnosis
The diagnosis of a patient with suspected syphilis must begin with a detailed history (including sexual behavior, a risk assessment for HIV and other STDs and questioning regarding symptoms) and a physical examination (including examination of the skin, scalp, oropharynx, genital and anal area and a targeted neurological examination). In certain settings, direct darkfield microscopy or fluorescent antibody testing of lesion exudates can be performed. Serological testing is used to confirm the diagnosis in those with signs and symptoms or as a screening test in those who are asymptomatic. Non-treponemal testing (rapid plasma reagin [RPR], venereal disease research laboratory [VDRL], toluidine red unheated serum test [TRUST]) is used as the initial screen followed by a treponemal test (treponemal pallidum particle agglutination [TP-PA]) to confirm an initial reactive non-treponemal test. Increasingly in the United States and in other countries, due to the automation of treponemal testing using enzyme immunoassays, a treponemal antibody test is used as the initial syphilis screening test. Since treponemal antibodies persist for life in most patients with syphilis, the clinician cannot determine based on a positive treponemal test the duration of infection. A second non-treponemal test is then used and, if positive, the patient is diagnosed with recent syphilis infection. In some cases, however, the initial treponemal test will be reactive and the second non-treponemal test will be negative, making the determination of active infection difficult. If a patient has no history of syphilis treatment, most national guidelines recommend treatment in that setting for latent infection. Interpretation of all serological tests should be the same for both HIV-infected and HIV-uninfected patients [11]. In low-resource settings rapid treponemal tests are often used as the single assay. Persons with a reactive test are treated without determination of the serological titer and staged by history and clinical findings. Those with symptoms are categorized as early syphilis (primary or secondary) and those without symptoms as latent syphilis. If syphilis exposure is likely to have occurred within the past 12 months, the patient is managed as early syphilis; if the exposure was more than 12 months or unknown, the patient is managed as late syphilis.
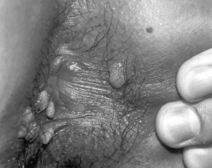
In patients with genital ulcerative lesions consistent with syphilis, other causes might include herpes simplex virus infection and chancroid. Rarely granuloma inguinale (Klebsiella granulomatis) is a cause of genital ulcer disease. Raised genital lesions of secondary syphilis (see Fig. 36.3) might also be confused with external genital warts Drug reactions, trauma, pyogenic bacterial infections, and malignancy might also be included in the differential diagnosis of early symptomatic syphilis. Because syphilis is curable, all patients with such genital lesions should be tested for syphilis [12, 13].
As congenital syphilis can be almost entirely avoided by early detection and treatment of maternal infections, all pregnant women should undergo syphilis screening at the first antenatal care visit [3]. Pregnant women with a reactive serology should be treated for latent syphilis if asymptomatic and early syphilis if there are clinical signs of disease. Male partners of pregnant women with syphilis should be treated presumptively to reduce the risk of maternal re-infection. Routine and frequent testing of syphilis in high-risk populations has been shown to be associated with a reduction in syphilis incidence in the target population [4].
Patients with signs or symptoms of neurologic involvement (e.g. cranial nerve palsies, motor or sensory defects) should undergo lumbar puncture and CSF examination to exclude neurosyphilis. Neurosyphilis is diagnosed on the basis of elevated cerebrospinal fluid total white cell count ([5-10] 10 cells/mm3 for HIV-uninfected patients and [10-20] 20 cells/mm3 for HIV-infected), abnormal protein or reactive VDRL or flourescent treponemal antibody-absorption test. Other indications for CSF analysis in patients with syphilis are suspected treatment failure or evidence of tertiary syphilis. HIV co-infection is not an indication for CSF analysis [11]. Because intramuscular penicillin G benzathine does not reach adequate levels in the brain to be treponemocidal, patients with neurosyphilis must be treated with intravenous penicillin G.
Treatment
Both HIV-infected and HIV-uninfected patients should undergo the same treatment: intramuscular benzathine penicillin G (see Table 36.1). The efficacy of benzathine penicillin G in the treatment of syphilis has been support by decades of clinical experience and numerous case series, observational studies, and clinical trials. Patients should be informed about the Jarisch–Herxheimer reaction—fever, headache, chills, and rigors—which is an acute febrile reaction that usually occurs within the first 24 hours after syphilis treatment. It most often occurs among patients who have early syphilis and is more frequent among HIV-infected patients [7]. Pretreatment with acetaminophen (paracetamol) can reduce the severity of that reaction. In many countries, it is recommended that the treatment of pregnant women occurs in a hospital setting because the Jarisch–Herxheimer reaction is associated with premature labor and fetal loss.
Table 36.1 Recommended treatment regimens for syphilis patients [11, 12]
| Population | Recommended regimens | Comments |
|---|---|---|
| Primary, secondary, and early latent syphilis (first year of latent syphilis) | Benzathine penicillin G 2.4 million units administered intramuscularly (IM) as a single dose | Routine penicillin allergy screening with injectable penicillin in or under the skin prior to treatment administration is not recommended |
| Late latent syphilis, latent syphilis of unknown duration, or tertiary syphilis without CNS involvement | Benzathine penicillin G 2.4 million units administered IM at 1-week intervals for three weeks for a total of 7.2 million units | Pregnant women who miss any dose must repeat the full course of therapy. In the general population if more than 14 days lapse between doses then the full course of therapy should be repeated [3] |
| Neurosyphilis or syphilitic eye disease | Intraveneous aqueous crystalline penicillin G 3–4 million units every 4 hours or continuous infusion for 10–14 days (total 18–24 million units per day) followed by at least one dose of benzathine penicillin G IM, 2.4 million units | |
| Childrena with primary, secondary, or early latent syphilis | Benzathine penicillin G 50,000 units/kg IM, up to the adult dose of 2.4 million units | For children ≥ 1 month with acquired primary or secondary syphilis conduct an evaluation through consultation with child-protection services and a CSF examination for detection of asymptomatic neurosyphilis |
| Children with late latent syphilis or latent syphilis of unknown duration | Benzathine penicillin G 50,000 units/kg IM, up to the adult dose of 2.4 million units, administered at -week intervals for three weeks (3 doses in total) |
a Treatment for congenital syphilis is more complex and beyond the scope of this chapter.
Some efficacy has been demonstrated with other antibiotics in patients who have true penicillin allergies; for example, doxycycline, 100 mg orally twice a day for 14 days. However, tetracyclines are contraindicated in pregnant women and children < 8 years of age. Pregnant women with penicillin allergies should be desensitized and treated with penicillin [11]. A series of congenital syphilis cases was reported among pregnant women with syphilis in China treated with azithromycin [14]. A randomized controlled trial comparing intravenous ceftriaxone to intravenous penicillin G in HIV-infected patients with neurosyphilis found similar improvements clinically and in laboratory measures such as the CSF VDRL titer, CSF white blood cell count, and CSF protein concentration for the two groups [15].
Treatment success for both HIV-uninfected and HIV-infected persons is defined as a four fold decrease in nontreponemal titers at 6 to 12 months post treatment in early syphilis and 12 to 24 months post treatment in late syphilis. HIV-infected patients should be re-evaluated every 3 months for a year after initial treatment [11]. When considering treatment failure, the possibility of re-infection should be considered, as this simply requires retreatment. In the absence of evidence of re-infection, CSF analysis should be performed to rule out neurosyphilis, and a 3-week course of treatment should be initiated [4, 7]. If neurosyphilis cannot be excluded, the patient must be treated for neurosyphlis with intravenous penicillin G.
Genital Herpes
Epidemiology
Both herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) are prevalent worldwide. Most persons are infected with HSV-1 in childhood; however, HSV-2 infection is usually transmitted sexually and risk factors are thus similar to those of other sexually transmitted diseases: the number of lifetime sex partners, young age at sexual debut, and previous history of STDs [16]. HSV-1 seroprevalence in adults approaches 70% in developed countries and 100% in developing countries. Sub-Saharan Africa has the highest burden of HSV-2 infection, ranging from 20–80% in low-risk adults and about 40–90% in high-risk adults, with slightly lower prevalence in West Africa than the other regions. Similar prevalence of HSV-2 infection is found in Central and South America and the Middle East, ranging from about 30% to 50%. Although data are sparse from countries in Asia, available data suggest a lower HSV-2 prevalence [17].
Across the globe HSV-2 infection is more prevalent among women than men, and prevalence increases with age [17]. The burden of HSV-2 infection is consistently higher in HIV-infected individuals than in HIV-uninfected individuals. There is a wealth of epidemiologic evidence demonstrating that prevalent HSV-2 is associated with a two- to eight-fold increase in incident HIV-1 infection, even when adjusted for sexual behavior [18, 19]. Because HSV-2 infection leads to the disruption of the genital mucosa and an influx of CD4-bearing T cell lymphocytes, it is biologically plausible that HSV-2 infection could lead to increased risk of HIV-1 acquisition [18]. It has also been suggested that persons co-infected with HSV-2 and HIV-1 are more likely to transmit HIV-1; however, recent studies have found similar rates of HIV-1 transmission between HSV-2 infected and uninfected individuals [18]. Several clinical trials to treat HSV-2 infection were recently completed that demonstrated no impact of HSV-2 treatment on HIV acquisition or transmission [20, 21]. Treatment of HSV-2 infection in HSV-2/HIV co-infected patients, however, was associated with a delay in HIV disease progression [22].
Natural history, pathogenesis, and pathology
A person becomes infected with HSV after exposure to virus that is shed from the genital tract or skin of an HSV-infected individual. While most HSV-1 infection usually occurs from oral contact (kissing) in childhood, in many countries HSV-1 is increasingly being acquired from oral-genital contact in adolescence [23]. HSV-2 acquisition almost always occurs through sexual contact. Once infected, the virus replicates at the site of infection. The virus then spreads through the lymphatic system to the sensory nervous system, where it persists. HSV is cleared from the cells at the site of infection; however, recurrences at this and other sites can occur after HSV reactivation and transport back down the nerves to the dermis, skin, or mucous membranes. Reactivation of HSV leads to viral shedding, even in the absence of symptoms. Because most infections are asymptomatic, sexual transmission of herpes usually occurs from persons who are unaware they are infected [9, 16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree