These control both the development of the distinct male and female phenotype and affect behaviour and physiology thus ensuring that the gametes have an optimal chance of delivery and that mating occurs at the optimal time for fertilization. The sex hormones also prepare the female to carry the developing embryo throughout pregnancy and to nurture it through the period of lactation and dependency.
Differentiation into male and female
The indifferent embryo
State
Karyotype
Phenotype (Expressed Sex)
Incidence per Live Births
Notes and Effects
Normal female
46, XX
Female
Turner’s syndrome
45, X0
Female
0.1 per 1000 females
Females are usually short in stature, possibly with a broad chest, webbed neck, cubitus valgus (extreme outward displacement of the extended forearm) and autism. They are infertile (primary amenorrhoea) and sexually immature. Associated with younger mothers
‘Super female’
47, XXX
Female
1.0 per 1000
Normal in females appearance and fertility, may be mentally retarded
Normal male
46, XY
Male
Klinefelter’s syndrome
47, XXY (up to four X chromosomes have been found)
Male
1.3 per 1000 males
Affected males are tall and thin with long limbs and small tests. May be infertile (azoospermia) and have gynaecomastia (breast development). May be mentally retarded. More common in sons of older mothers
‘Super male’
47, XYY
Male
1.0 per 1000 males
Affected males tend to be tall, have reduced IQ and show ‘antisocial’ behaviour. Some studies show increased incidence (2–3%) in institutes for the criminally insane
Sex reversed
46, XXsxr
Male
1.0 per 20 000
Small piece of Y chromosome containing SRY gene is translocated on to an X chromosome
The undifferentiated gonad
The development of the male morphology
Embryological development
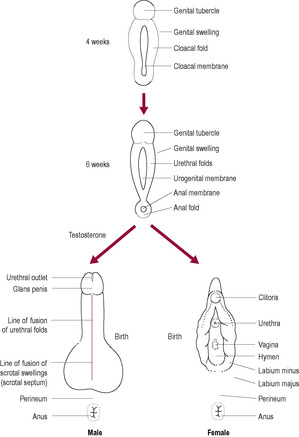
Fig. 5.4
Puberty
The development of the female morphology
Embryological development
Common abnormalities
Puberty: the initiation of fertility cycles
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Sexual differentiation and behaviour
Zara and her husband, James, would very much like to know the sex of their babies and had been informed by one of their friends, who happens to be a doctor and who they met while in Africa, that they would be able to find out the sex when they have the 12-week ultrasound scan.
• When Zara informs you, her midwife, that they wish to be told the sex of the babies when the scan is performed, what do you think would be important to discuss with Zara and her husband regarding the identification of the sex of the babies?
• Are there any situations where it can be justified to identify the sex of the baby at the 12-week scan?
• What should the midwife do if a woman requests a termination of pregnancy solely because she knows the sex of her baby?
Sexual dimorphism is genetically controlled in many animals. However, in some species, such as crocodiles and tortoises, sexual dimorphism is principally determined by environment. The temperature of incubation of the egg promotes the development of either male or female offspring. Intermediate temperatures of egg incubation result in the ratios of the sexes altering in relation to the differing temperature gradients. In birds, the female carries the ZW chromosomes and the male the ZZ chromosomes, whereas some species of fish are able to change sex during a single lifetime. Differentiation of the sexes in mammals also involves sexual dimorphism of the urinary system because the two systems are closely linked in their development.
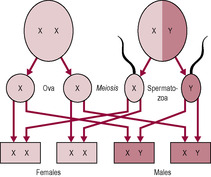
In humans, differentiation into either a male or female fetus is almost always under genetic control, depending on whether the ovum is fertilized by a sperm carrying an X chromosome (gynosperm; female) or a Y chromosome (androsperm; male). However, events such as maternal pyrexia in the first trimester of pregnancy can result in abnormal cell division and development, in some cases contributing to indeterminate (ambiguous) sexual characteristics as well as other physical malformations. As in all mammals, the human female is the homogametic sex and usually carries the XX chromosome arrangement whereas the male is the heterogametic sex and carries the XY arrangement for reproductive function to be successful. Therefore, it is the sperm that determines the sex of the fetus (Fig. 5.1). Rarely, mosaic individuals (carrying a patchwork of XX and XY cells) or those individuals with mutations in genes important for sex determination may express a phenotype opposite to their karyotype leading to XX males or XY females. If a Y chromosome is present, the individual develops testes (male gonads) regardless of the number of X chromosomes. The Y chromosome is much smaller than the X chromosome. The DNA of the Y chromosome is very condensed and so incapable of synthesizing RNA (see Chapter 7). Essentially, the Y chromosome switches on or controls the other genes required for testes formation; these genes are on the other autosomal chromosomes and the X chromosome.
The development of the fetus, both male and female, is initially the same. The gonads are formed from the mesenchymal tissue of the genital ridge primordia which develop each side of the descending aorta. Until approximately the 4th week of gestation, the fetus is in a sexually undifferentiated state. After this phase, the differentiation process is initiated by the activation of the SRY (sex-determining region of the Y) gene, usually found only upon the Y chromosome (Sinclair et al., 1990). The SRY gene triggers a complex cascade of events leading to testicular development (Vilain, 2000) either by activating genes leading to male development or inhibiting a repressor of male development. SRY is also expressed in a number of brain structures and may be involved in sexual behaviour. If the SRY gene is not activated (even though the genotype is XY), the female morphological form will develop. Occasionally, the SRY gene may be translocated on to an X gene, so if it is activated a male morphological state may develop from an XX genotype. Other genes are involved in SRY gene activity; it seems that the ratio of these genes to SRY is more important than the absolute amount of the genes (Johnson, 2007).
Abnormal numbers of sex chromosomes are often compatible with fetal development, and therefore occur with a relatively high birth frequency (Table 5.1). The major consequence of an aberrant number of X chromosomes is infertility. If there is an additional one or more X chromosome, as in Klinefelter’s syndrome (47 chromosomes, XXY), the fetus will differentiate along the male pathway, as the Y chromosome is present. The absence of a Y chromosome, as in normal female development (XX) or Turner’s syndrome (45 chromosomes, X0, where 0 indicates an absent sex chromosome), will result in the fetus developing as a female.
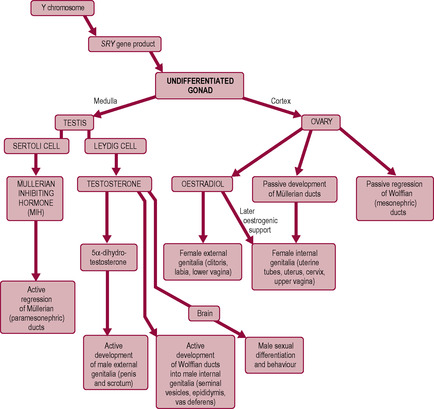
The factors that activate the SRY gene remain unknown; however, its effects are orchestrated through its influence on the production of androgens. The effects of these hormones upon tissue differentiation and development result in sexual dimorphism of the male during the embryonic phase. During the embryonic phase, female form develops in the absence of endocrine activity although oestrogen is required for puberty and fertility. Therefore, a genetic male may develop female characteristics if the SRY gene is either absent or not activated (Fig. 5.2).
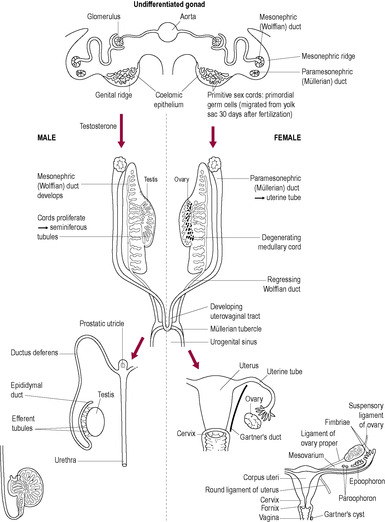
The early human embryo is bipotential at all levels of sexual differentiation. The gonads are derived from three embryonic tissue sources: the coelomic epithelium, the underlying mesenchyme and the primordial germ cells (PGCs; Fig. 5.3). The coelomic epithelium develops into the genital ridge, which is found on the medial side of the mesonephros (which develops from the mesenchyme). The primitive germ cells, which are ultimately responsible for the production of the gametes (spermatozoa and ova), originate from the yolk sac. Here, they undergo rapid mitosis before migrating from the yolk sac wall towards the genital ridge, about 4 weeks after fertilization. The genital ridges appear to produce chemotactic substances that attract the primitive germ cells, stimulating them to develop pseudopodia and undergo amoeboid movement. Colonization of the primitive gonad by the PGCs is completed during the 6th week of embryonic development. The primitive sex cords develop from the gonadal ridges into the underlying mesenchyme forming the medulla and cortex of the gonad. In the testes, the medulla develops and will go on to form Sertoli cells and the cortex regresses; this is reversed in the development of the ovary where the cells of the sex cords condense into clusters around the PGCs (oogonia) and go on to form the primordial ovarian follicles. Two sets of primitive internal genitalia begin development. Further development will follow either the male or the female route depending on the hormonal influences.
The embryo has two sets of primitive unipotential internal genitalia, each of which has the potential to develop depending on the hormonal environment. The SRY gene and the male gonad are essential for the development of male morphology. The SRY gene stimulates the medulla of the undifferentiated gonad to develop into the testes and produce two hormones, testosterone and anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting substance, MIS or Müllerian-inhibiting hormone, MIH), which promote male genital duct development. In the absence of AMH and testosterone secretion, female sexual differentiation occurs. AMH, from Sertoli cells, drives the regression of the Müllerian structures (paramesonephric ‘female’ ducts) and testosterone, from Leydig cells, stimulates development of the Wolffian (mesonephric ‘male’) ducts into the male internal genitalia, the epididymis, vas deferens and seminiferous tubules. In the absence of testosterone, the Wolffian structures regress and the Müllerian ducts continue to develop into the uterus, uterine tubes and the upper part of the vagina. Sexual differentiation along the male pathway requires active diversion, whereas differentiation into a female embryo follows an inherent pattern or ‘default pathway’. However, some genes have been recognized as important in ovarian development (Goodfellow and Camerino, 1999).
The Wolffian, or mesonephric, ducts initially develop as part of the embryological renal system. The adaptation of the mesonephric ducts to form the male morphology is a significant development in sexual dimorphism, in evolutionary terms. Sexual differentiation at this stage is very efficient; it is extremely rare for individuals to have both testicular and ovarian tissues. These true hermaphrodites often have an internal testis on one side and an ovary on the other side (or a mixed structure known as ovatestis) which may be a result of chimerism (the fusion of a male and female embryo; Strain et al., 1998).
The phenotypic sex is determined by the sexual characteristics of the individual. The external genitalia are bipotential (can become either male or female) and initially exist as a urogenital slit flanked by urethral folds, a genital swelling and a genital tubercle or bud. Steroid hormones directly influence the development of male external genitalia (unlike the female). Testosterone from the testes is converted into 5α-dihydrotestosterone (5α-DHT) within the target cells. Under the influence of this biologically more potent androgen, the tissues of the external genitalia form the penis and scrotum (Fig. 5.4). The urethral folds fuse enclosing the urethral tube to form the shaft of the penis and genital swellings fuse to form the scrotum. The genital tubercle expands to form the glans penis. The testes, like the ovaries, initially develop within the abdominal cavity but do not remain there. They descend to their normal position within the scrotal sac, suspended outside the abdominal cavity, just before or soon after birth. However, it is quite common (in ~1 in 50 live-born males) for either one or both testes to fail to descend at this time (the condition is described as cryptorchidism). Spontaneous descent usually occurs within the first year of life. Testicular damage, potentially resulting in later failure of spermatogenesis and a higher incidence of malignant tumours, occurs if the testes remain within the abdominal cavity, so the testes are surgically lowered (orchiopexy or orchidopexy) if spontaneous resolution has not occurred (Thorup and Cortes, 2009).
(Adapted with permission from Johnson and Everitt, 1995.)
Testosterone is also converted into oestrogens within the brain. The presence of oestrogen is believed to be responsible for differentiation of certain brain structures along a male or female pathway. This resulting difference in morphology underpins the biological explanations for behavioural patterns differing between the sexes. Male sexual activity appears to depend on the presence of testosterone above a critical threshold. Female sexual activity in the human may be cyclical in response to changes in male behaviour. There are cyclical changes in the organic acid content of vaginal secretions (derived from normal bacterial flora), which may be a mechanism of olfactory communication to the woman’s sexual partner (see Chapter 4).
The male embryo produces testosterone with a peak of about 2 ng/mL at about weeks 13–15 (Johnston, 2007). Levels fall from then but peak to about the same level about 3 months after birth. Thereafter levels fall but slowly increase from about 12 months. As in the female, puberty commences when the secretory pattern of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), under the influence of gonadotrophin-releasing hormone (GnRH), becomes mature. Initially, secretion of LH increases nocturnally which explains the pattern of nocturnal sperm emission in pubertal boys. FSH and LH orchestrate spermatogenesis within the male (see Chapter 2). At puberty, the testes increase in size as the seminiferous tubules canalize, the Sertoli cells increase in size and the germ cells resume mitotic activity. Unlike the ovarian cycle, spermatogenesis is a continuous process resulting in the production of many gametes. The testes produce testosterone from the Leydig cells, which influences the development of the male secondary sex characteristics (Box 5.1). Unlike the female, the male retains the capability of spermatogenesis indefinitely but failure to achieve copulation becomes more common with the progression of age (Corona et al., 2010) and overall production of testosterone gradually falls from the 4th decade onwards (Schill, 2001).
Box 5.1
• Enlargement of the penis
• Pubic and axillary hair growth
• Deepening of voice (due to growth of larynx)
• Masculine pattern of fat distribution
• Development of the skeletal muscle (protein anabolism)
• Secretion of skin oil glands (predisposes to acne)
• Bone growth and adolescent growth spurt (via growth hormone secretion)
• Male sexual behaviour and aggression
As the X chromosome does not contain the SRY gene, the Müllerian ducts differentiate into the female internal genitalia, the uterine tubes and fimbriae, the uterus, cervix and upper two-thirds of the vagina. The undifferentiated gonad develops into the ovary; the cortex develops and the medulla regresses. This is the route of differentiation in the absence of testosterone and MIH. Female external genitalia form independently of any hormonal influences; therefore, the ovary has little endocrine activity until puberty. The genital tubercle becomes the clitoris and the urethral folds and genital swellings remain unfused, forming the labia minora and majora, respectively.
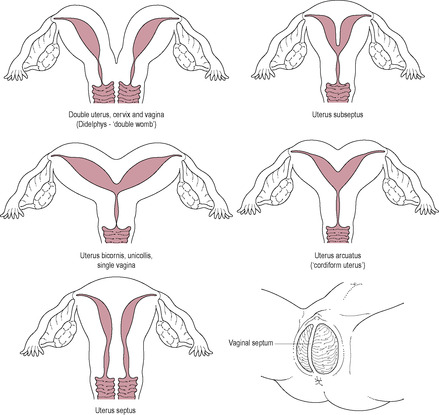
During development, the body of the uterus, cervix and upper vagina are formed by the fusion of the two Müllerian (paramesonephric) ducts. Abnormalities may range from a simple uterine septum to the complete duplication of the reproductive system (Fig. 5.5). The failure of one of the paramesonephric ducts to develop will result in a unilateral rudimentary horn.
In the female, the germ cells or oogonia cease mitotic division and enter their first meiotic division (becoming primary oocytes) and most of them die before birth so the number of oocytes a woman has is finite and determined before her birth. Meiotic division is then arrested until the oocyte is triggered to resume development. Recruitment of primordial follicles into the pool of developing follicles begins at puberty. Puberty commences with the activation of the hypothalamus to produce GnRH in a mature pattern of secretion. It is suggested that the menarche is initiated when a critical mass of body fat, which may be genetically defined, is accumulated (Frisch, 1990). GnRH stimulates the anterior pituitary to produce FSH and LH, which orchestrate the reproductive cycles in the female (see Chapter 4). The ovaries begin to produce oestrogens, which influence the development of the female secondary sex characteristics. The breasts develop, the deposition of adipose tissue is responsible for the distinct female body curvature, and the growth of hair in the axilla and genital region commences.
Get Clinical Tree app for offline access