Respiratory Regulation of Carbon Dioxide and Fluids
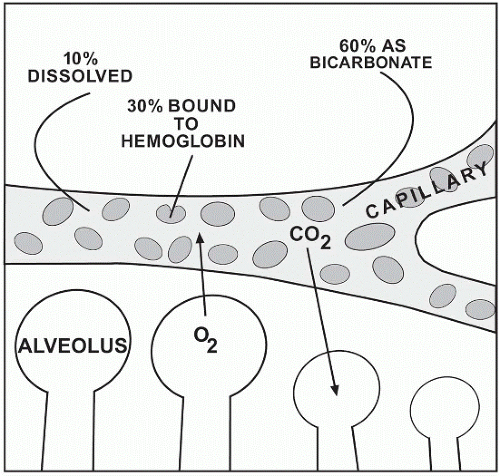
Through the process of breathing, a combination of oxygen and nitrogen gases enters the respiratory system. As the gases travel the vascular system, carbon dioxide travels the opposite pathway in exchange for oxygen. Carbon dioxide diffuses into the capillary at the venous end and returns to the lungs via the pulmonary artery and arterioles. Once in the pulmonary bed, it diffuses across the alveolar-capillary interspace to enter the alveolus for expiration. Approximately 10% of carbon dioxide travels in its dissolved state in plasma. It is the dissolved CO2 that is measured as the pCO2 with a range of 35-45 mm Hg. This range closely resembles that of the partial pressure of gases in the alveoli. Any increase in the amount of CO2 easily can tilt the pH balance to an acidic state. Just as easily, any decrease in CO2 can result in an increase in alkalinity. Of the remaining carbon dioxide, 30% is attached to hemoglobin and 60% is transported as bicarbonate. The dissolved CO2 is carried to the alveoli of the lungs while the remaining diffuses into tissue spaces and capillaries.
Chloride Shift
Carbon dioxide prefers to combine with hemoglobin in the red blood cell or with carbonic acid. As mentioned previously, the H2CO3– easily ionizes to HCO3– and H+. The hydrogen ion is allowed to combine with hemoglobin, Hb–, to form hydrogen hemoglobin, HHb, a buffer for acid-base alterations. If large amounts of bicarbonate are drawn into the plasma and a negative charge is needed in the red blood cell for equalization, Cl– moves into the cell. This movement, referred to as a chloride shift, allows for increased amount of bicarbonate to move into the plasma to act as a buffering mechanism while chloride maintains electrical neutrality within the cell.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree