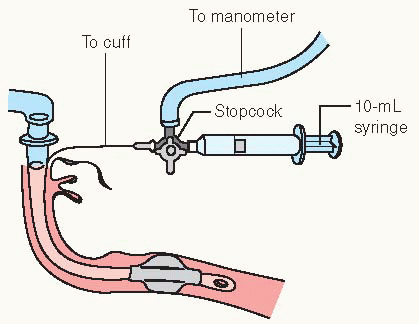
Alveolus—air sac where gas exchange takes place.
Apex—top portion of the upper lobes of lungs.
Base—bottom portion of lower lobes of lungs, located just above the diaphragm.
Bronchoconstriction—constriction of smooth muscle surrounding bronchioles.
Bronchus—large airways; lung divides into right and left bronchi.
Carina—location of division of the right and left main stem bronchi.
Cilia—hair-like projections on the tracheobronchial epithelium, which aid in the movement of secretions and removal of debris.
Compliance—ability of the lungs to distend and change in volume relative to an applied change in pressure (eg, emphysema— lungs very compliant; fibrosis—lungs noncompliant or stiff).
Dead space—ventilation that does not participate in gas exchange; also known as wasted ventilation when there is adequate ventilation but no perfusion, as in pulmonary embolus or pulmonary vascular bed occlusion. Normal dead space is 150 mL.
Diaphragm—primary muscle used for respiration; located just below the lung bases, it separates the chest and abdominal cavities.
Diffusion (of gas)—movement of gas from area of higher to lower concentration.
Dyspnea—subjective sensation of breathlessness associated with discomfort, often caused by a dissociation between motor command and mechanical response of the respiratory system as in:
Respiratory muscle abnormalities (hyperinflation and airflow limitation from chronic obstructive pulmonary disease [COPD]).
Abnormal ventilatory impedance (narrowing airways and respiratory impedance from COPD or asthma).
Abnormal breathing patterns (severe exercise, pulmonary congestion or edema, recurrent pulmonary emboli).
Arterial blood gas (ABG) abnormalities (hypoxemia, hypercarbia).
Hemoptysis—the expectoration (coughing-up) of blood or of blood-stained sputum from the larynx, trachea, bronchi, or lungs.
Hypoxemia—PaO2 less than normal, which may or may not cause symptoms. (Normal PaO2 is 80 to 100 mm Hg on room air.)
Hypoxia—insufficient oxygenation at the cellular level due to an imbalance in oxygen delivery and oxygen consumption. (Usually causes symptoms reflecting decreased oxygen reaching the brain and heart.)
Mediastinum—compartment between lungs containing lymph and vascular tissue that separates left from right lung.
Orthopnea—shortness of breath when in reclining position.
Paroxysmal nocturnal dyspnea—sudden shortness of breath associated with sleeping in recumbent position.
Perfusion—blood flow, carrying oxygen and CO2 that passes by alveoli.
Pleura—serous membrane enclosing the lung; comprised of visceral pleura, covering all lung surfaces, and parietal pleura, covering chest wall and mediastinal structures, between which exists a potential space.
Pulmonary circulation—network of vessels that supply oxygenated blood to and remove CO2-laden blood from the lungs.
Pulmonary hypertension—an increase in blood pressure in the pulmonary artery, pulmonary vein, or pulmonary capillaries.
Respiration—inhalation and exhalation; at the cellular level, a process involving uptake of oxygen and removal of CO2 and other products of oxidation.
Shunt—adequate perfusion without ventilation, with deoxygenated blood conducted into the systemic circulation, as in pulmonary edema, atelectasis, pneumonia, COPD.
Surfactant—fluid secreted by alveolar cells that reduces surface tension of pulmonary fluids and aids in elasticity of pulmonary tissue.
Ventilation—is the process by which oxygen and CO2 are transported to and from the lungs
Ventilation-perfusion (V/Q) imbalance or mismatch— imbalance of ventilation and perfusion; a cause for hypoxemia. V/Q mismatch can be due to:
Blood perfusing an area of the lung where ventilation is reduced or absent.
Ventilation of parts of lung that are not perfused.
 Evidence Base
Evidence Base
Characteristics—Is the dyspnea acute or chronic? Has it occurred suddenly or gradually? Is more than one pillow required to sleep? Is the dyspnea progressive, recurrent, or paroxysmal? Walking how far leads to shortness of breath? How does it compare to the patient’s baseline level of dyspnea? Ask patient to rate dyspnea on a scale of 1 to 10, with 1 being no dyspnea and 10 being the worst imaginable. What relieves and what aggravates the dyspnea?
Associated factors—Is there a cough associated with the dyspnea and is it productive? What activities precipitate the shortness of breath? Does it seem to be worse when upset? Is it influenced by the time of day, seasons, and/or certain environments? Does it occur at rest or with exertion? Any fever, chills, night sweats, ankle/leg swelling? Any change in body weight?
History—Is there a patient history or family history of chronic lung disease, cardiac or neuromuscular disease, cancer, problems with blood clotting, or immunocompromise? What is the smoking history?
Significance—Sudden dyspnea could indicate pulmonary embolus, pneumothorax, myocardial infarction (MI), acute heart failure, or acute respiratory failure. In a postsurgical or postpartum patient, dyspnea may indicate pulmonary embolus or edema. Orthopnea can be indicative of heart disease or COPD. If dyspnea is associated with a wheeze, consider asthma, COPD, heart failure, or upper airway obstruction. When dyspnea occurs in combination with fatigue, pulmonary hypertension may exist. Metabolic disorders, psychiatric conditions, and neuromuscular disorders may also contribute to dyspnea.
Characteristics—Is the pain sharp, dull, stabbing, or aching? Is it intermittent or persistent? Is the pain localized or does it radiate? If it radiates, where? How intense is the pain? Are there factors that alleviate or aggravate the pain, such as position or activity?
Associated factors—What effect do inspiration and expiration have on the pain? What other symptoms accompany the chest pain? Is there diaphoresis, shortness of breath, nausea?
History—Is there a smoking history or environmental exposure? Has the pain ever been experienced before? What was the cause? Is there a preexisting pulmonary or cardiac diagnosis? Has there been recent trauma?
Significance—Chest pain related to pulmonary causes is usually felt on the side where pathology arises, but it can be referred. Dull persistent pain may indicate carcinoma of the lung, whereas sharp, stabbing pain usually arises from the pleura. Dyspnea with pleuritic chest pain indicates clinically significant pulmonary embolism.
 Evidence Base
Evidence Base
Characteristics—Is the cough dry, hacking, loose, barky, wheezy, or more like clearing the throat? Is it strong or weak? How frequent is it? Is it worse at night or at any time of day? Does the intensity change on days off from work? Is there seasonal variation? Is it aggravated by food intake or exertion. Is it alleviated by any medication? How long has it been going on?
Associated factors—Is the cough productive? If so, what is the consistency, amount, color, and odor of the sputum? How does sputum compare to the patient’s baseline? Is it associated with shortness of breath, pain, or nausea?
History—Is there a smoking history? Is the smoking current or in the past? Has there been any environmental or occupational exposure to dust, fumes, or gases that could lead to cough? Are there past pulmonary diagnoses, asthma, rhinitis, allergy, or exposure to allergens, such as pollen, house dust mites, animal dander, birds, mold or fungi, cockroach waste, irritants (smoke, odors, perfumes, cleaning products, exhaust, pollution, cold air)? Has there been prolonged exposure to dampness, chemical sanitizers, cobalt or other hard metals, beryllium, asbestos, dusts from coal, wood, or grains? Does the patient have a history of acid reflux or use an angiotensinconverting enzyme inhibitor with a common adverse effect of cough? Has there been a concurrent voice change? Has the patient recently traveled outside the country? Can the patient identify any specific triggers?
Significance—A dry, irritative cough may indicate viral respiratory tract infection. A cough at night should alert to potential left-sided heart failure, asthma, or postnasal drip worsening at night. A morning cough with sputum might be bronchitis. A cough that is less severe on days off from work may be related to occupational or environmental exposures. A patient with severe or changing cough should be evaluated for bronchogenic carcinoma. Consider bacterial pneumonia if sputum is rusty, and lung tumor if it is pink-tinged. A profuse pink frothy sputum could be indicative of pulmonary edema. A cough associated with food intake could indicate problems with aspiration. A dry cough may be associated with pulmonary fibrosis. History of recent travel may be associated with infection from a source not commonly identified in the United States.
Characteristics—Is the blood from the lungs? It could be from the GI system (hematemesis) or upper airway (epistaxis). Is it bright red and frothy? How much? Is onset associated with certain circumstances or activities? Was the onset sudden, and is it intermittent or continuous?
Associated factors—Was there an initial sensation of tickling in the throat? Was there a salty taste or burning or bubbling sensation in the chest? Has there been shortness of breath, chest pain, difficulty with exertion?
History—Was there any recent chest trauma or respiratory treatment (chest percussion)? Does the patient have an upper respiratory infection, sinusitis, or recent epistaxis? Has the patient used cocaine or other illicit drugs?
Significance—Hemoptysis can be linked to pulmonary infection, lung carcinoma, abnormalities of the heart or blood
vessels, pulmonary artery or vein abnormalities, or pulmonary emboli and infarction. Small amounts of blood-tinged sputum may be from the upper respiratory tract, and regurgitation of blood comes from a GI bleed.
 Evidence Base
Evidence Base
What is the respiratory rate, depth, and pattern? Are accessory muscles being used? Is the patient breathing through the mouth or pursing lips during exhalation? Is sputum being raised, and what is its appearance and odor?
Is there an increase in the anterior to posterior chest diameter, suggesting air trapping?
Is there obvious orthopnea or splinting?
Is there clubbing of the fingers, associated with bronchiectasis, lung abscess, empyema, cystic fibrosis, pulmonary neoplasms, and various other disorders?
Is there central cyanosis indicating possible hypoxemia or cardiac disease? Are mucous membranes and nail beds pink?
Are there signs of tracheal deviation, as seen with pneumothorax?
Are the jugular veins distended? Is there peripheral edema or other signs of cardiac dysfunction?
Does palpation of the chest cause pain? Is chest expansion symmetrical? Any change in tactile fremitus?
Is percussion of lung fields resonant bilaterally? Is diaphragmatic excursion equal bilaterally?
Are breath sounds present and equal bilaterally? Are the lung fields clear or are there rhonchi, wheezing, crackles, stridor, or pleural friction rub? Does auscultation reveal egophony, bronchophony, or whispered pectoriloquy?
A measurement of O2, CO2, and the pH of the blood that provides a means of assessing the adequacy of ventilation (PaCO2), metabolic status (pH), and oxygenation (PaO2).
Allows assessment of body’s acid-base (pH) status, indicating if acidosis or alkalosis is present, whether acidosis or alkalosis is respiratory or metabolic in origin, and whether it is compensated or uncompensated.
Used for diagnostic evaluation and evaluation of response to clinical interventions (oxygen therapy, mechanical ventilation, etc.).
Blood can be obtained from any artery but is usually drawn from the radial, brachial, or femoral site. It can be drawn directly by arterial puncture or accessed by way of indwelling arterial catheter (see Procedure Guidelines 10-1, pages 200 to 202). Determine facility policy for qualifications for ABG sampling and site of arterial puncture.
If the radial artery is used, an Allen test must be performed before the puncture to determine if collateral circulation is present.
Arterial puncture should not be performed through a lesion, through or distal to a surgical shunt, or in area where peripheral vascular disease or infection is present.
Coagulopathy or medium- to high-dose anticoagulation therapy may be a relative contraindication for arterial puncture.
Results may be affected by recent changes in oxygen therapy, suctioning, or positioning.
Interpret ABG values by looking at trends for the patient as well as the following normal values:
PaO2—partial pressure of arterial oxygen (80 to 100 mm Hg)
PaCO2—partial pressure of arterial carbon dioxide (35 to 45 mm Hg)
SaO2—arterial oxygen saturation (>95%)
pH—hydrogen ion concentration, or degree of acid-base balance (7.35 to 7.45); bicarbonate (HCO3–) ion primarily a metabolic buffer (22 to 26 mEq/L).
Sputum may be obtained for evaluation of gross appearance, microscopic examination, Gram stain, culture and sensitivity, acid-fast bacillus, and cytology.
The direct smear shows presence of white blood cells and intracellular (pathogenic) bacteria and extracellular (mostly nonpathogenic) bacteria.
Gram stain shows whether bacteria is gram-positive or gram-negative and can be used to guide therapy until culture and sensitivity results are available.
The sputum culture is used to identify presence of specific pathogens; sensitivity determines drug efficacy and serves as a guide for drug treatment (ie, choice of antibiotic).
Acid-fast smears detect presence of pathogens such as Mycobacterium tuberculosis.
Cytology identifies abnormal and possibly malignant cells.
Patients receiving antibiotics, steroids, and immunosuppressive agents for a prolonged time may have periodic sputum examinations because these agents may give rise to opportunistic pulmonary infections.
It is important that the sputum be collected correctly and that the specimen be sent to a laboratory immediately. Allowing it to stand in a warm room will result in overgrowth of organisms, making identification of pathogen difficult; this also alters cell morphology. A series of three early morning specimens is needed for acid-fast bacillus examination. Cytology samples should be collected in container with fixative solution.
Sputum can be obtained by various methods:
Deep breathing and coughing.
An early morning specimen obtained prior to eating or drinking yields best sample of deep pulmonary secretions from all lung fields.
Have patient clear nose and throat and rinse mouth with plain water—to decrease contamination by oral and upper respiratory flora.
Position patient upright or in high Fowler’s unless contraindicated. Instruct patient to take several deep breaths, exhale, and perform a series of short coughs.
Have patient cough deeply and expectorate the sputum into a sterile container.
If patient is unable to produce specimen, increasing oral fluid intake may be useful (unless on fluid restriction).
Induction through use of ultrasonic or hypertonic saline nebulization.
Patient inhales mist through mouth slowly and deeply for 10 to 20 minutes.
Nebulization increases the moisture content of air going to lower tract; particles will condense on tracheobronchial tree and aid in expectoration.
Suctioning—aspiration of secretions via mechanical means; must be used with caution because it may cause bleeding, cardiac dysrhythmias, and increased intracranial pressure.
Tracheal, through endotracheal (ET) or tracheostomy tube.
Nasotracheal (NT), through nose and into back of throat or trachea.
Bronchoscopy with bronchoalveolar lavage, in which 60 to 100 mL is instilled and aspirated from various lung segments. Sedation and/or anesthesia is required.
Gastric aspiration (rarely necessary since advent of ultrasonic nebulizer).
Nasogastric (NG) tube is inserted into the stomach, approximately 50 mL sterile water is instilled, and swallowed pulmonary secretions are siphoned out.
Useful only for culture of tubercle bacilli, not for direct examination.
Transtracheal aspiration involves passing a needle and then a catheter through a percutaneous puncture of the cricothyroid membrane. Transtracheal aspiration bypasses the oropharynx and avoids specimen contamination by mouth flora.
Generally, a sputum sample of 15 mL is adequate for laboratory testing.
Commercially available blood gas kit or
2-or 3-mL syringe
23G or 25G needle
0.5 mL sodium heparin (1:1,000)
Stopper or cap
Sterile germicide
Cup or plastic bag with crushed ice
Gloves
Goggles
Rolled towel or washcloth
PROCEDURE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pleural fluid is continuously produced and reabsorbed, with a thin layer normally in the pleural space. Abnormal pleural fluid accumulation (effusion) occurs in diseases
of the pleura, heart, or lymphatics. The pleural fluid is studied, with other tests, to determine the underlying cause.
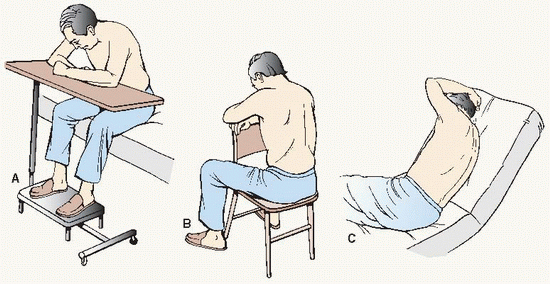
Obtained by aspiration (thoracentesis) or by tube thoracotomy (chest tube insertion; see Procedure Guidelines 10-2).
The fluid is examined for cancerous cells, cellular makeup, chemical content, and microorganisms.
Pleural cavity usually contains less than 20 mL clear yellow (serous) fluid that lubricates the surfaces of the pleura, the thin membrane that lines the chest cavity and surrounds
the lungs. A pleural effusion is an abnormal collection of this fluid.
The test is performed to determine the cause of a pleural effusion, and to relieve associated shortness of breath.
Thoracentesis tray (if available) or
5-, 20-, 50-mL syringes
Needles: 22G, 26G, or 16G (3 inches long)
Three-way stopcock and tubing
Hemostat
Biopsy needle
Germicide solution
Local anesthetic (such as lidocaine 1%)
Sterile gauze pads (4” × 4” and 2” × 2”)
Sterile towels and drape
Sterile specimen containers
Sterile gloves
Overhead table and chair
PROCEDURE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Observe and record total amount of fluid withdrawn, nature of fluid, and its color and viscosity.
Prepare sample of fluid and ensure transport to the laboratory.
A chest x-ray may be done before or after the fluid is withdrawn.
Patient should not cough, breathe deeply, or move while fluid is being withdrawn.
Instruct patient to inform provider immediately if sharp chest pain or shortness of breath occurs.
Normal pulmonary tissue is radiolucent and appears black on film. Thus, densities produced by tumors, foreign bodies, and infiltrates can be detected as lighter or white images.
Commonly, two views—posterior—anterior and lateral—are obtained.
This test shows the position of normal structures, displacement, and presence of abnormal shadows. It may reveal pathology in the lungs in the absence of symptoms.
Should be taken upright if patient’s condition permits. Assist technician at bedside in preparing patient for portable chest x-ray.
Encourage patient to take deep breath, hold breath, and remain still as x-ray is taken.
Make sure that all jewelry, electrocardiogram (ECG) or monitor leads, and metal objects (including metal-containing transdermal patches) in x-ray field are removed so as not to interfere with film.
Consider the contraindication of x-rays for pregnant patients.
Cross-sectional x-rays of the lungs are taken from many different angles and processed through a computer to create three-dimensional images. This three-dimensional imaging provides more complete diagnostic information than the twodimensional x-ray.
It may be used to define pulmonary nodules and pulmonary abnormalities or to demonstrate mediastinal abnormalities and hilar adenopathy.
Describe test to patient and family, including that table will slide into doughnut-shaped scanner and patient must lie still during test. The test usually takes about 30 minutes, but may take longer.
Be alert to allergies to iodine or other radiographic contrast media that might be used during testing.
For scans performed with contrast, informed consent and IV access required. Patient should take nothing by mouth (NPO) for 4 hours prior to scan. Blood urea nitrogen and creatinine should be evaluated 24 to 48 hours before test.
Hydrate patient well to facilitate excretion of contrast, if used.
Check regarding weight limit of equipment prior to scanning bariatric patients.
Consider the contraindication of radiologic studies for the pregnant patient, especially computed tomography (CT) scans with contrast media.
Noninvasive procedure that uses a powerful magnetic field, radio waves, and a computer to produce detailed pictures of organs, soft tissue, bone, and other internal structures.
Provides contrast between various soft tissues and is performed to:
Assess abnormal growths, including cancer of the lungs or other tissues inadequately assessed with other imaging modalities.
Determine stage of cancer, including tumor size, extent, and degree to which cancer has spread.
Visualize lymph nodes and blood vessels.
Assess disorders of the vertebrae, ribs, and sternum.
Traditional radiographic contrast media are not used but gadolinium injection or ingestion may be necessary, depending on patient’s medical history and anatomy to be imaged.
It is helpful to synchronize the magnetic resonance imaging (MRI) picture to the ECG in thoracic studies.
The hazards of MRI during pregnancy are unknown, although ionizing radiation (x-ray) is not used during MRI.
Explain procedure to patient and assess ability to remain still in a closed space; sedation may be necessary if the patient is claustrophobic. The test takes approximately 1 hour.
Evaluate patient for contraindications to MRI: implanted devices, such as implanted defibrillators that may malfunction; cochlear implants; or metallic surgical clips used on brain aneurysms.
Devices that may interfere with the exam or potentially pose a risk include artificial heart valves, implanted drug infusion ports, infusion catheters, intrauterine devices, pacemakers and neurostimulators, metallic implants such as prosthetic valves or joints, and metal pins, screws, plates, or surgical staples.
Make sure that all jewelry, ECG or monitor leads, hearing aids, pins/hairpins, removable dental work, and metal objects (including metal-containing transdermal patches) are removed.
Kidney disease and sickle cell anemia may contraindicate MRI with contrast material.
Check with MRI technician about the use of equipment, such as ventilator or mechanical IV pump, in MRI room.
Earplugs may be used to muffle loud thumping and humming noises during imaging.
Evaluate the patient for claustrophobia, and teach relaxation techniques to use during test or advocate for use of open MRI. Be ready to administer sedation, if necessary.
Check regarding size capacity of equipment prior to scanning bariatric patients.
It is recommended that nursing mothers not breastfeed for 36 to 48 hours after MRI with contrast.
Physiologic images are obtained, based on the detection of radiation from the emission of positrons. Positrons are tiny particles emitted from a radioactive isotope—administered IV to the patient. Radioactivity localizes in the area(s) being scanned and is detected by positron emission tomography (PET). Different colors or degrees of brightness on a PET image represent different levels of tissue or organ function.
Radioisotope is administered 30 to 90 minutes prior to scan via IV or inhalation.
Distinguishes between benign and malignant lung nodules.
Describe test to patient and family, including that table will slide into doughnut-shaped scanner and patient must lie still during test. The test usually takes 30 to 45 minutes.
Isotope has a short half-life and is not considered a radiologic hazard.
Encourage fluids to facilitate excretion of isotope.
Consider the contraindication of radiologic studies for the pregnant or breastfeeding patient.
Test results may be inaccurate for diabetic patients or for patients who have eaten within a few hours prior to the scan if blood glucose or insulin levels are not normal.
An imaging method used to study pulmonary vessels and pulmonary circulation.
For visualization, radiopaque medium is injected by way of a catheter in the main pulmonary artery rapidly into the vasculature of the lungs. Films are then taken in rapid succession after injection.
It is considered the “gold standard” for diagnosis of pulmonary embolus, but spiral CT scanning can also be effectively used.
Determine whether patient is allergic to radiographic contrast media, describe the procedure, and obtain informed consent.
Patient may be kept NPO for 4 to 8 hours prior to procedure.
Instruct patient that injection of dye may cause flushing, cough, and a warm sensation.
Pulse, blood pressure (BP), and respirations are monitored during the procedure.
After the procedure, make sure pressure is maintained over access site and monitor pulse rate, BP, and circulation distal to the injection site.
Patient may be advised to keep extremity straight for up to 12 hours after the procedure.
Necessity of test should be carefully evaluated in patients with bleeding disorders and pregnant women.
A pair of nuclear scan tests using inhaled and injected radioisotopes to measure breathing (ventilation) and blood flow (perfusion) in all areas of the lungs. The two tests may be performed either separately or together.
Perfusion scan is done after injection of a radioactive isotope. It measures blood perfusion through the lungs and evaluates lung function on a regional basis.
Ventilation scan is done after inhalation of radioactive gas (eg, xenon with O2), which diffuses throughout the lungs. It indicates how well air reaches all parts of the lung.
Usually performed to detect pulmonary embolus. Also useful in evaluating advanced pulmonary disease (COPD), detecting abnormal circulation (shunts) in the pulmonary blood vessels, evaluating lung function, and identifying fibrosis.
Is also referred to as V/Q scan because the initials are used in mathematical equations that calculate air and blood flow.
Determine if patient is allergic to radiographic dye before V/Q scan.
Explain the procedure to patient and encourage cooperation with inhalation and brief episodes of breath holding.
Contraindicated in patients with primary pulmonary hypertension.
False positives may occur in patients with vasculitis, mitral stenosis, pulmonary hypertension, and when tumors obstruct a pulmonary artery with airway involvement, fatty tissues, and presence of parasites.
False negatives are associated with partially occluded vessels.
The direct inspection and observation of the upper and lower respiratory tract through fiberoptic (flexible) or rigid bronchoscope as a means of diagnosing and managing inflammatory, infectious, and malignant diseases of the airway and lungs.
Flexible fiberoptic bronchoscopy allows for more patient comfort and better visualization of smaller airways, including nasal passages. It is usually performed using local anesthesia with or without moderate sedation. Fluoroscopy may be needed to facilitate specimen collection. Used for therapeutic and diagnostic procedures such as:
Bronchoalveolar lavage.
Endobronchial or transbronchial biopsies.
Cytologic wash or brush.
Transbronchial needle aspiration.
Endobronchial ultrasound.
Autofluorescence bronchoscopy.
Balloon dilation.
Endobronchial laser ablation.
Electrocautery.
Photodynamic therapy.
Brachytherapy.
Some types of stent placement.
Rigid bronchoscopy, often performed under general anesthesia with adequate sedation and muscle relaxants, may be combined with flexible bronchoscopy for better access to distal airways. Diagnostic and therapeutic indications include:
Bleeding or hemorrhage.
Foreign body extraction.
Deeper biopsy specimen collection than can be obtained fiber-optically.
Dilation of tracheal or bronchial strictures.
Relief of airway obstruction.
Insertion of stents.
Tracheobronchial laser therapy or other mechanical tumor ablation.
Check that an informed consent form has been signed and that risks and benefits have been explained to patient.
Make sure that IV access is present and patent.
Absolute contraindications include uncorrectable coagulopathy, severe refractory hypoxemia, unstable hemodynamic status. Patients with increased risk are those with MI within past 6 weeks, head injuries susceptible to increased intracranial pressures (ICP), and known or suspected pregnancy (due to possible radiation exposure).
Review and follow facility policy and procedure for anesthesia and sedation.
Administer prescribed medication to reduce secretions, block the vasovagal reflex and gag reflex, and relieve anxiety. Give encouragement and nursing support.
Restrict fluid and food, as ordered, before procedure to reduce risk of aspiration when reflexes are blocked. Patient may be kept NPO for 4 hours prior to flexible bronchoscopy with minimal sedation; if deeper sedation is used, patient’s time on NPO is extended.
Remove dentures, contact lenses, and other prostheses.
After the procedure:
Monitor cardiac rhythm and rate, BP, and level of consciousness (LOC).
Monitor respiratory effort and rate.
Monitor oximetry.
Withhold ice chips and fluids until patient demonstrates gag reflex.
Monitor patient’s perceptions of pain, discomfort, and dyspnea.
Promptly report cyanosis, hypoventilation, hypotension, tachycardia or dysrhythmia, hemoptysis, dyspnea, decreased breath sounds.
Provide outpatients with specific instructions regarding signs and symptoms of complications and what to do if they arise.
 NURSING ALERT
NURSING ALERT
Procedures used for obtaining histologic material from the lung to aid in diagnosis include:
Transbronchial biopsy—biopsy forceps inserted through bronchoscope and specimen of lung tissue obtained.
Transthoracic needle aspiration biopsy—specimen obtained through needle aspiration under fluoroscopic guidance.
Open lung biopsy—specimen obtained through small anterior thoracotomy; used in making a diagnosis when other biopsy methods have not been effective or are not possible.
Obtain permission for consent, if required.
Observe for possible complications, including pneumothorax, hemorrhage (hemoptysis), and bacterial contamination of pleural space.
A chest x-ray should be done 1 hour after transbronchial biopsy to exclude pneumothorax.
See “Bronchoscopy” (page 207) or “Thoracic Surgeries” (page 263) for postprocedure care.
Pulmonary function tests (PFTs) are used to detect and measure abnormalities in respiratory function and quantify severity of various lung diseases. Such tests include measurements of lung volumes, ventilatory function, diffusing capacity, gas exchange, lung compliance, airway resistance, and distribution of gases in the lung.
Ventilatory studies (spirometry) are the most common group of tests.
Requires electronic spirometer, water spirometer, or wedge spirometer that plots volume against time (timed vital capacity).
Patient is asked to take as deep a breath as possible and then to exhale into spirometer as completely and either slowly or as forcefully as possible, depending on the type of test.
Results are compared with normals for patient’s age, height, and sex (see Table 10-1).
A reduction in the vital capacity, inspiratory capacity, and total lung capacity may indicate a restrictive form of lung disease (disease due to increased lung stiffness).
An increase in functional reserve capacity, total lung capacity, and reduction in flow rates usually indicate an obstructive flow due to bronchial obstruction or loss of lung elastic recoil.
Lung volumes are determined by asking the patient to inhale a known concentration of inert gas, such as helium or 100% oxygen, and measuring concentration of inert gas or nitrogen in exhaled air (dilution method) or by plethysmography.
Yields thoracic volume (total lung capacity, plus any unventilated blebs or bullae).
An increased residual volume is found in air-trapping due to obstructive lung disease.
A reduction in several parameters usually indicates a restrictive form of lung disease or chest wall abnormality.
Diffusing capacity measures lung surface effective for the transfer of gas in the lung by having patient inhale gas containing known low concentration of carbon monoxide and measuring carbon monoxide concentration in exhaled air. Difference between inhaled and exhaled concentrations is related directly to uptake of carbon monoxide across alveolarcapillary membrane. Diffusing capacity is reduced in interstitial lung disease, emphysema, pneumonectomy, pulmonary embolus, and anemia.
Instruct patient in correct technique for completing PFTs; coach patient through test, if needed. Test results may be unreliable if patient is not cooperative or motivated to perform all maneuvers.
Table 10-1 Pulmonary Function Tests
TERM
SYMBOL
DESCRIPTION
REMARKS
Vital capacity
VC
Maximum volume of air exhaled after a maximum inspiration
• VC < 10-15 mL/kg suggests need for mechanica ventilation
• VC > 10-15 mL/kg suggests ability to wean
Forced vital capacity
FVC
Vital capacity performed with a maximally forced expiratory effort
• Reduced in obstructive disease (COPD) due to air-trapping and restrictive disease
• Reflects airflow in large airways
Forced expiratory volume in 1 second
FEV1
Volume of air exhaled in the first second of the performance of the FVC
• Reduced in obstructive disease (COPD) due to air-trapping
• Reflects airflow in large airways
Ratio of FEV1/FVC
FEV1/FVC
FEV1 expressed as a percentage of the FVC
• Decreased in obstructive disease
• Normal in restrictive disease
Forced midexpiratory flow
FEF25%-75%
Average flow during the middle half of the FVC
Reflects airflow in small airways
• Smokers may have change in this test before other symptoms develop
Peak expiratory flow rate
PEFR
Most rapid flow during a forced expiration after a maximum inspiration
• Used to measure response to bronchodilators, airflow obstruction in patients with asthma
Maximal voluntary volume
MV V
Volume of air expired in a specified period (12 seconds) during repetitive maximal effort
• An important factor in exercise tolerance
• Decreases in neuromuscular diseases
Instruct patient not to use oral or inhaled bronchodilator (eg, albuterol), caffeine, or tobacco at least 4 to 6 hours before test (longer for long-acting bronchodilators).
Used with caution in patients with hemoptysis of unknown origin; pneumothorax; unstable cardiovascular status; recent MI or pulmonary embolus; thoracic, abdominal, or cerebral aneurysm; or recent eye, thoracic, or abdominal surgery.
Using a noseclip for all spirometric maneuvers is strongly recommended.
Although forced expiratory volume in the first second of a forced vital capacity is the “gold standard” for diagnosing airway obstruction, there is no single cut-off value separating normal from abnormal. This determination is based more on trending of individual results.
Noninvasively provides an estimate of arterial oxyhemoglobin saturation by using selected wavelengths of light to determine the saturation of oxyhemoglobin. Oximeters function by passing a light beam through a vascular bed, such as the finger or earlobe, to determine the amount of light absorbed by oxygenated (red) and deoxygenated (blue) blood.
Calculates the amount of arterial blood that is saturated with oxygen (SaO2) and displays this as a percentage.
Provides indication only of oxygenation, not ventilation.
Indications include:
Monitor adequacy of oxygen saturation; quantify response to therapy.
Monitor unstable patient who may experience sudden changes in blood oxygen level.
Evaluation of need for home oxygen therapy.
Determine supplemental oxygen needs at rest, with exercise, and during sleep.
Need to follow the trend and need to decrease number of ABG sample drawn.
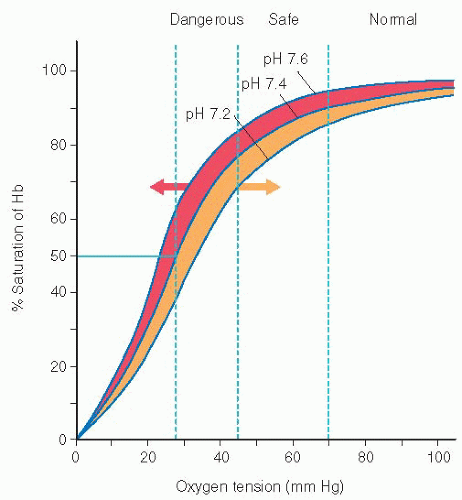
The oxyhemoglobin dissociation curve allows for correlation between SaO2 and PaO2 (see Figure 10-1).
Increased body temperature, acidosis, and increased phosphates (2,3-DPG) cause a shift in the curve to the right, thus increasing the ability of hemoglobin to release oxygen to the tissues.
Decreased temperature, decreased 2,3-DPG, and alkalosis cause a shift to the left, causing hemoglobin to hold on to the oxygen, reducing the amount of oxygen being released to the tissues.
Increased bilirubin, increased carboxyhemoglobin, low perfusion, or SaO2 less than 80% may alter light absorption and interfere with results.
 NURSING ALERT
NURSING ALERT
Assess patient’s hemoglobin. SaO2 may not correlate well with PaO2 if hemoglobin is not within normal limits.
Remove patient’s nail polish because it can affect the ability of the sensor to correctly determine oxygen saturation, particularly polish with blue or dark colors.
Correlate oximetry with ABG values and then use for single reading or trending of oxygenation (does not monitor PaCO2).
Display heart rate should correlate with patient’s heart rate.
To improve quality of signal, hold finger dependent and motionless (motion may alter results) and cover finger sensor to occlude ambient light.
Assess site of oximetry monitoring for perfusion on a regular basis because pressure ulcer may occur from prolonged application of probe. Rotate probe every 2 hours.
Device limitations include motion artifact, abnormal hemoglobins (carboxyhemoglobin and methemoglobin), intravenous (IV) dye, exposure of probe to ambient light, low perfusion states, skin pigmentation, nail polish or nail coverings, and nail deformities such as severe clubbing.
Document inspired oxygen or supplemental oxygen and type of oxygen delivery device.
Accuracy can be affected by decreased peripheral perfusion, ambient light, IV dyes, nail polish, deeply pigmented skin, cold extremities, hypothermia, patients in sickle cell crisis, jaundice, severe anemia, and use of antibiotics such as sulfas.
In patients with COPD, oxygen saturation levels may remain unchanged, even though CO2 levels may be rising as the patient becomes acidotic. Pulse oximetry will not detect this deterioration.
Contraindicated for monitoring patients who have high levels of arterial carboxyhemoglobin, such as fire victims.
 Evidence Base
Evidence Base
Used to noninvasively determine and monitor end-tidal carbon dioxide (ETCO2)—the amount of CO2 that is expired with each breath—via colorimetric indicator.
ETCO2 is displayed as a capnogram (a waveform that may be time-based or volume-based and a numeric reading).
Normally 2 to 5 mm Hg less than PaCO2 in adults, with the difference being greater in the presence of lung disease, or increase in dead space, and can be used as an indirect estimate of V/Q mismatching for the lung.
Indicated for monitoring severity of pulmonary disease and monitoring response to therapy; evaluating efficacy of mechanical ventilatory support; monitoring adequacy of pulmonary, systemic, and coronary blood flow; and assessing metabolic rate and/or alveolar ventilation.
A standard in anesthesia care, it is being increasingly used in the critical care setting as well as in emergency care via pocket-sized models.
Draw ABGs initially to correlate ETCO2 with PaCO2 and to establish the gradient between PaCO2 and ETCO2.
Accuracy of measurement can be affected by alterations in breathing pattern or tidal volume (VT), breathing frequency, presence of freon from metered-dose inhalers, contamination of the system by secretions or condensate, low cardiac output, use of antacids or carbonated beverages, and leaks around tracheal tube cuffs or uncuffed tubes.
Does not evaluate pH or oxygenation.
Effective for confirming ET tube placement and for monitoring CO2 in patients who tend to retain CO2 (eg, COPD).
Not a reliable method for determining inadvertent pulmonary placement of gastric tubes.
Clean and disinfect sensors and monitors as per manufacturer’s instructions.
 Evidence Base
Evidence Base
Emerging as a source for determining biomarkers of lung disease. Exhaled breath condensate (EBC) is a matrix of exhaled particles and droplets in which biomarkers may be identified, such as:
Volatile (acetic acid, formic acid, ammonia) and nonvolatile compounds.
Very-low- and low-molecular-weight compounds.
Polypeptides.
Proteins.
Nucleic acids.
Lipid mediators.
Inorganic molecules.
Organic molecules.
Redox relevant molecules.
pH relevant molecules.
Cytokines and chemokines.
Currently, there is no gold standard—either invasively or noninvasively—for determining absolute concentrations with which EBC can be easily compared.
The pH of EBC depends on disease state, ranging from 3-5 to 9.0, which can affect the reactivity and stability of other biomarkers being assayed.
Collect condensate sample. Collection methods vary, depending on specific biomarkers to be analyzed. Ten minutes of tidal breathing yields 1 to 2 mm of sample; smaller sample sizes may be adequate for analysis of only one or two biomarkers.
Be aware that lower airway condensate may be contaminated by particles from oral and retropharyngeal mucosa.
Saliva trapping systems may be used with drooling patients to reduce salivary contamination.
Oropharyngeal airway—curved plastic device inserted through the mouth and positioned in the posterior pharynx to move tongue away from palate and open the airway.
Usually for short-term use in the unconscious patient or may be used along with an oral ET tube.
Not used if recent oral trauma, surgery, or if loose teeth are present.
Does not protect against aspiration.
Nasopharyngeal airway (nasal trumpet)—soft rubber or plastic tube inserted through nose into posterior pharynx.
Facilitates frequent nasopharyngeal suctioning.
Use extreme caution with patients on anticoagulants or bleeding disorders.
Select size that is slightly smaller than diameter of nostril and slightly longer than distance from tip of nose to earlobe.
Check nasal mucosa for irritation or ulceration, and clean airway with hydrogen peroxide and water.
Laryngeal mask airway—composed of a tube with a cuffed masklike projection at the distal end; inserted through the mouth into the pharynx; seals the larynx and leaves distal opening of tube just above glottis.
Easier placement than ET tube because visualization of vocal cords is not necessary.
Provides ventilation and oxygenation comparable to that achieved with an ET tube.
Cannot prevent aspiration because it does not separate the GI tract from the respiratory tract.
May cause laryngospasm and bronchospasm.
Combitube—double-lumen tube with pharyngeal lumen and tracheoesophageal lumen; pharyngeal lumen has blocked distal end and perforations at pharyngeal level; tracheoesophageal lumen has open upper and lower end; large oropharyngeal balloon serves to seal mouth and nose; distal cuff seals the esophagus or trachea.
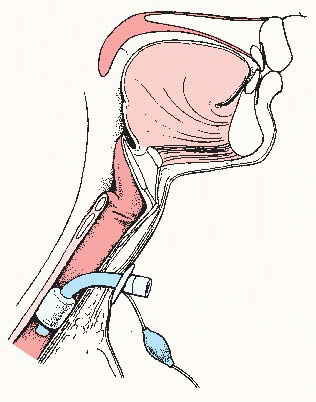
Endotracheal tube—flexible tube inserted through the mouth or nose and into the trachea beyond the vocal cords that acts as an artificial airway.
Maintains a patent airway.
Allows for deep tracheal suction and removal of secretions.
Permits mechanical ventilation.
Inflated balloon seals off trachea so aspiration from the GI tract cannot occur.
Generally easy to insert in an emergency, but maintaining placement is more difficult so this is not for long-term use.
Tracheostomy tube—firm, curved artificial airway inserted directly into the trachea at the level of the second or third tracheal ring through a surgically made incision.
Permits mechanical ventilation and facilitates secretion removal.
Can be for long-term use.
Bypasses upper airway defenses, increasing susceptibility to infection.
Allows the patient to eat and swallow.
Orotracheal insertion is technically easier because it is done under direct visualization (see Procedure Guidelines 10-3, pages 212 to 214). Disadvantages are increased oral secretions, decreased patient comfort, difficulty with tube stabilization, and inability of patient to use lip movement as a communication means.
NT insertion may be more comfortable to the patient and is easier to stabilize. Disadvantages are that blind insertion is required; possible development of pressure necrosis of the nasal airway, sinusitis, and otitis media.
Tube types vary according to length and inner diameter, type of cuff, and number of lumens.
Usual sizes for adults are 6.0, 7.0, 8.0, and 9.0 mm.
Most cuffs are high volume, low pressure, with self-sealing inflation valves, or the cuff may be of foam rubber.
Most tubes have a single lumen; however, dual-lumen tubes may be used to ventilate each lung independently (see Figure 10-2).
May be contraindicated when glottis is obscured by vomitus, bleeding, foreign body, or trauma or cervical spine injury or deformity.
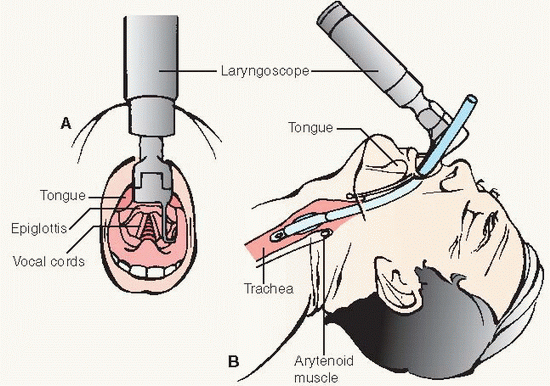
Laryngoscope with curved or straight blade and working light source (check batteries and bulb regularly)
Endotracheal (ET) tube with lowpressure cuff and adapter to connect tube to ventilator or resuscitation bag
Stylet to guide the ET tube
Oral airway (assorted sizes) or bite block to keep patient from biting into and occluding the ET tube
Adhesive tape or tube fixation system
Sterile anesthetic lubricant jelly (water-soluble)
10-mL syringe
Suction source
Suction catheter and tonsil suction
Resuscitation bag and mask connected to oxygen source
Sterile towel
Gloves
Face shield
End tidal CO2 detector
PROCEDURE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tube types vary according to presence of inner cannula and presence and type of cuff (see Figure 10-3, page 216).
Tubes with high-volume, low-pressure cuffs with selfsealing inflation valves; with or without inner cannula.
Fenestrated tube.
Foam-filled cuffs.
Speaking tracheostomy tube.
Tracheal button or Passy-Muir valve.
Silver tube (rarely used).
Vary according to length and inner diameter in millimeters. Usual sizes for an adult are 5.0, 6.0, 7.0, and 8.0.
Tracheostomy is usually planned, either as an adjunct to therapy for respiratory dysfunction or for longer-term airway management when ET intubation has been used for more than 14 days.
May be done at the bedside in an emergency when other means of creating an airway have failed (see Procedure Guidelines 10-4, pages 216 to 218).
Acute respiratory failure, central nervous system (CNS) depression, neuromuscular disease, pulmonary disease, chest wall injury.
Upper airway obstruction (tumor, inflammation, foreign body, laryngeal spasm).
Anticipated upper airway obstruction from edema or soft tissue swelling due to head and neck trauma, some postoperative head and neck procedures involving the airway, facial or airway burns, decreased LOC.
Need for airway protection (vomiting, bleeding, or altered mental status).
Aspiration prophylaxis.
Fracture of cervical vertebrae with spinal cord injury; requiring ventilatory assistance.
Laryngeal or tracheal injury.
Sore throat, hoarse voice.
Glottic edema.
Trauma (damage to teeth or mucous membranes, perforation or laceration of pharynx, larynx, or trachea).
Aspiration.
Laryngospasm, bronchospasm.
Ulceration or necrosis of tracheal mucosa.
Vocal cord ulceration, granuloma, or polyps.
Vocal cord paralysis.
Postextubation tracheal stenosis.
Tracheal dilation.
Formation of tracheal-esophageal fistula.
Formation of tracheal—arterial fistula.
Innominate artery erosion.
Pulmonary infection and sepsis.
Dependence on artificial airway.
Tracheostomy tube (sizes 6-9 mm for most adults)
Sterile instruments: hemostat, scalpel and blade, forceps, suture material, scissors
Sterile gown and drapes, gloves
Cap and face shield
Antiseptic prep solution
Gauze pads
Shave prep kit
Sedation
Local anesthetic and syringe
Resuscitation bag and mask with oxygen source
Suction source and catheters
Syringe for cuff inflation
Respiratory support available for post-tracheostomy (mechanical ventilation, tracheal oxygen mask, CPAP, T-piece)
PROCEDURE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ensure adequate ventilation and oxygenation through the use of supplemental oxygen or mechanical ventilation as indicated.
Assess breath sounds every 2 hours. Note evidence of ineffective secretion clearance (rhonchi, crackles), which suggests need for suctioning.
Provide adequate humidity when the natural humidifying pathway of the oropharynx is bypassed.
Provide adequate suctioning of oral secretions to prevent aspiration and decrease oral microbial colonization.
Use clean technique when inserting an oral or nasopharyngeal airway, and take it out and clean it with hydrogen peroxide and rinse with water at least every 8 hours.
Perform frequent oral care with soft toothbrush or swabs and antiseptic mouthwash or hydrogen peroxide diluted with water. Frequent oral care will aid in prevention of ventilatorassociated pneumonia. The patient’s lips should be kept moisturized with petroleum jelly to prevent them from becoming sore and cracked.
Ensure that aseptic technique is maintained when inserting an ET or tracheostomy tube. The artificial airway bypasses the upper airway, and the lower airways are sterile below the level of the vocal cords.
Elevate the patient to a semi-Fowler’s or sitting position, when possible; these positions result in improved lung compliance. The patient’s position, however, should be changed at least every 2 hours to ensure ventilation of all lung segments and prevent secretion stagnation and atelectasis. Position changes are also necessary to avoid skin breakdown.
If an oral or nasopharyngeal airway is used, turn patient’s head to the side to reduce the risk of aspiration (because there is no cuff to seal off the lower airway).
Consciousness is usually impaired in patient with an oropharyngeal airway, so oral feeding is contraindicated.
To enhance comfort, remove a nasopharyngeal airway in the conscious patient during mealtimes.
Recognize that an ET tube holds the epiglottis open. Therefore, only the inflated cuff prevents the aspiration of oropharyngeal contents into the lungs. The patient must not receive oral feeding. Administer enteral tube feedings or parenteral feedings, as ordered.
Administer oral feedings to a conscious patient with a tracheostomy, usually with the cuff inflated. The inflated cuff prevents aspiration of food contents into the lungs, but causes the tracheal wall to bulge into the esophageal lumen, and may make swallowing more difficult.
Patients who are not on mechanical ventilation and are awake, alert, and able to protect the airway are candidates for eating with the cuff deflated.
To assess ability to protect the airway, sit the patient upright and feed the patient colored gelatin or juice. If color from gelatin can be suctioned from the tracheostomy tube, aspiration is occurring, and the cuff must be inflated during feeding and for 1 hour afterward with head of bed elevated.
Patients should receive thickened rather than regular liquids; this will assist in effective swallowing.
 NURSING ALERT
NURSING ALERT
ET tube cuffs should be inflated continuously and deflated only during intubation, extubation, and tube repositioning.
Tracheostomy tube cuffs also should be inflated continuously in patients on mechanical ventilation or continuous positive airway pressure (CPAP).
Tracheostomized patients who are breathing spontaneously may have the cuff inflated continuously (in the patient with decreased LOC without ability to fully protect airway), deflated continuously, or inflated only for feeding if the patient is at risk of aspiration.
Monitor cuff pressure every 4 hours (see Procedure Guidelines 10-5, pages 220 to 222).
Secure an ET tube so it cannot be disrupted by the weight of ventilator or oxygen tubing or by patient movement.
Use strips of adhesive tape or Velcro straps wrapped around the tube and secured to tape on patient’s cheeks or around the back of patient’s head.
Replace when soiled or insecure or when repositioning of tube is necessary.
Position tubing so traction is not applied to ET tube.
Perform tracheostomy site care at least every 8 hours using hydrogen peroxide and water, and change tracheostomy ties at least once per day (see Procedure Guidelines 10-6, pages 223 to 224).
Make sure ventilator or oxygen tubing is supported so traction is not applied to the tracheostomy tube.
Have available at all times at the patient’s bedside a replacement ET tube in the same size as patient is using, resuscitation bag, oxygen source, and mask to ventilate the patient in the event of accidental tube removal. Anticipate your course of action in such an event.
ET tube—know location and assembly of reintubation equipment including replacement ET tube. Know how to contact someone immediately for reintubation.
Tracheostomy—have extra tracheostomy tube, obturator, and hemostats at bedside. Be aware of reinsertion technique, if facility policy permits, or know how to contact someone immediately for reinserting the tube.
 NURSING ALERT
NURSING ALERT
Assist patient to deal with psychological aspects related to artificial airway.
Recognize that patient is usually apprehensive, particularly about choking, inability to communicate verbally, inability to remove secretions, uncomfortable suctioning, difficulty in breathing, or mechanical failure.
Explain the function of the equipment carefully.
Inform patient and family that speaking will not be possible while the tube is in place, unless using a tracheostomy tube with a deflated cuff, a fenestrated tube, a Passy-Muir speaking valve, or a speaking tracheostomy tube.
A Passy-Muir valve is a speaking valve that fits over the end of the tracheostomy tube. Air that is inhaled is exhaled through the vocal cords and out through the mouth, allowing speech.
Develop with patient the best method of communication (eg, sign language, lip movement, letter boards, paper and pencil, magic slate, or coded messages).
Patients with tracheostomy tubes or nasal ET tubes may effectively use orally operated electrolarynx devices.
Devise a means for patient to get the nurse’s attention when someone is not immediately available at the bedside, such as call bell, hand-operated bell, or rattle.
Anticipate some of patient’s questions by discussing “Is it permanent?” “Will it hurt to breathe?” “Will someone be with me?”
If appropriate, advise patient that as condition improves, a tracheostomy button may be used to plug the tracheostomy site. A tracheostomy button is a rigid, closed cannula that is placed into the tracheostomy stoma after removal of a cuffed or uncuffed tracheostomy tube. When in proper position, the button does not extend into the tracheal lumen. The outer edge of the button is at the skin surface and the inner edge is at the anterior tracheal wall (see Procedure Guidelines 10-7).
Suction catheter
Tonsil suction
Suction source
10-mL syringe
Pressure manometer (mercury or aneroid)
Handheld resuscitation bag with reservoir, connected to 100% O2 at 10-15 L/minute
Face shield
PROCEDURE | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Key Decision Point
Key Decision Point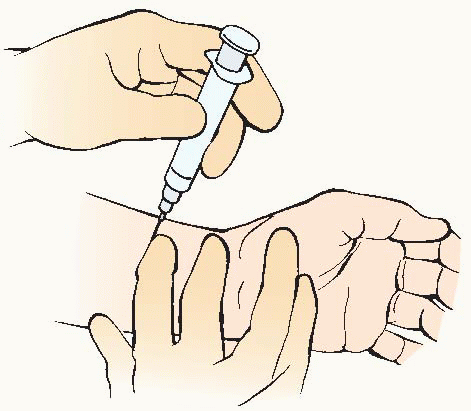
 Evidence Base DeWaay, D., & Gordon, J. (2011). The ABC’s of ABGs: Teaching arterial blood gases to adult learners. MedEdPORTAL Available: www.mededportal.org/publication/9038.
Evidence Base DeWaay, D., & Gordon, J. (2011). The ABC’s of ABGs: Teaching arterial blood gases to adult learners. MedEdPORTAL Available: www.mededportal.org/publication/9038.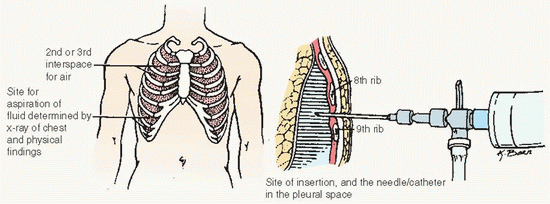


 Evidence Base Ahrens, T., Prentice, D., & Kleinpell, R. (2010). Critical care nursing certification: Preparation, review and practice exam (6th ed.). New york: McGraw-Hill.
Evidence Base Ahrens, T., Prentice, D., & Kleinpell, R. (2010). Critical care nursing certification: Preparation, review and practice exam (6th ed.). New york: McGraw-Hill.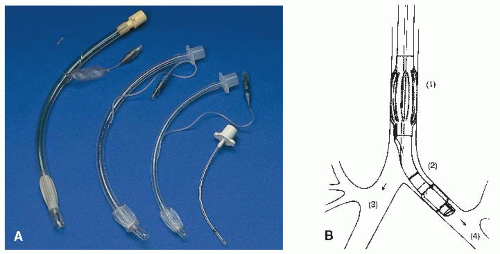

 KEY DECISION POINT
KEY DECISION POINT NURSING ALERT Accidental dislodgement of the tube could result when the ties are loose, and tube reinsertion through the as-yet-unformed stoma may be difficult or impossible to accomplish.
NURSING ALERT Accidental dislodgement of the tube could result when the ties are loose, and tube reinsertion through the as-yet-unformed stoma may be difficult or impossible to accomplish. Evidence Base Ahrens, T., Prentice, D., & Kleinpell, R. (2010). Critical care nursing certification: Preparation, review and practice exam (6th ed.). New York: McGraw-Hill.
Evidence Base Ahrens, T., Prentice, D., & Kleinpell, R. (2010). Critical care nursing certification: Preparation, review and practice exam (6th ed.). New York: McGraw-Hill.