OVERVIEW AND ASSESSMENT
In 2010, diabetes mellitus was estimated to affect almost 26 million Americans. This is more than 8% of the U.S. population. Unfortunately, this number is expected to double, if not triple, by 2050.
It is thought that the factors contributing to the explosion of diabetes are an aging population, increased prevalence of type 2 diabetes mellitus in youth, growing numbers of ethnic minorities, and the increasing trend of obesity (>65% of American adults are overweight or obese).
The cost of caring for people with diabetes is staggering. In 2007, the United States spent $174 billion on diabetes care alone. This is two to three times more than the health care costs of those without diabetes. By 2030, it is estimated that the United States will spend more than $333 billion on diabetes mellitus.
Diabetes mellitus is the leading cause of blindness, kidney failure, and lower limb amputations. People with diabetes are two to four times more likely to be diagnosed with heart disease, three times more likely to have dental disease, and twice as more likely to suffer from depression.
This is a very serious disease with even more serious consequences. All nurses must stay current on the latest care recommendations. Screening for this disease is essential because early treatment leads to a reduction of morbidity and mortality.
Insulin Secretion and Function
Insulin is a hormone secreted by the beta cells of the islet of Langerhans in the pancreas.
Small amounts of insulin are released into the bloodstream in response to changes in blood glucose levels throughout the day (basal secretion).
Increased secretion or a bolus of insulin, released during meals, helps maintain euglycemia.
Through an internal feedback mechanism that involves the pancreas and the liver, circulating blood glucose levels are maintained at a normal range of 60 to 100 mg/dL.
Insulin is essential for the utilization of glucose for cellular metabolism as well as for the proper metabolism of protein and fat.
Carbohydrate metabolism—insulin affects the conversion of glucose into glycogen for storage in the liver and skeletal muscles, and allows for the immediate release and utilization of glucose by the cells.
Protein metabolism—amino acid conversion occurs in the presence of insulin to replace muscle tissue or to provide needed glucose (gluconeogenesis).
Fat metabolism—storage of fat in adipose tissue and conversion of fatty acids from excess glucose occurs only in the presence of insulin.
Glucose can be used in the endothelial and nerve cells without the aid of insulin.
Without insulin, plasma glucose concentration rises and glycosuria results.
Absolute deficits in insulin result from decreased production of endogenous insulin by the beta cell of the pancreas.
Relative deficits in insulin are caused by inadequate utilization of insulin by the cell.
Classification of Diabetes
American Diabetes Association (ADA). (2013). Standards of medical care in diabetes (position statement). Diabetes Care, 36(Suppl. 1), S11-S66.
Type 1 Diabetes Mellitus
Type 1 diabetes mellitus (T1DM) was formerly known as insulindependent diabetes mellitus and juvenile diabetes mellitus.
Little or no endogenous insulin, requiring injections of insulin to control diabetes, prevent ketoacidosis and sustain life.
Only 5% to 10% of all people with diabetes have T1DM.
Etiology is not well understood: includes autoimmune, viral, and certain histocompatibility antigens as well as genetic components.
Clinical manifestation is abrupt with classic symptoms of polydipsia, polyphagia, polyuria, and weight loss.
Most commonly seen in patients under age 30 but can be seen in older adults.
Type 2 Diabetes Mellitus
Type 2 diabetes mellitus (T2DM) was formerly known as non-insulin-dependent diabetes mellitus or adult-onset diabetes mellitus.
Caused by a combination of insulin resistance and relative insulin deficiency—some individuals have predominantly insulin resistance, whereas others have predominantly deficient insulin secretion, with little insulin resistance.
Approximately 90% to 95% of people with diabetes have T2DM.
Etiology: strong hereditary component, commonly associated with obesity.
Usual presentation is slow and typically insidious with symptoms of fatigue, weight loss, poor wound healing, and recurrent infection.
Found primarily in adults over age 30; however, it is now more frequently seen in younger adults and adolescents who are overweight.
People with this type of diabetes may be treated with insulin, but are still referred to as having T2DM.
Gestational Diabetes Mellitus
Gestational diabetes mellitus (GDM) is defined as the onset of carbohydrate intolerance that initially presents during pregnancy and resolves upon delivery.
Affects approximately 2% to 10% of pregnancies; however, the new diagnostic criteria may yield that as many as 18% of pregnancies are affected by GDM.
Women with GDM have a 35% to 60% chance of developing T2DM diabetes in the next 10 to 20 years. They should be screened for diabetes 6 to 12 weeks postpartum and continue to have lifelong screening at least every 3 years.
GDM is associated with significant risk of maternal and fetal complications.
Due to the worldwide increases of both obesity and diabetes rates, the American Diabetes Association (ADA) made the recommendation that all pregnant women should be screened for the presence of T2DM at the initial prenatal visit using the standard diagnostic criteria (2011). Women who are negative for T2DM at that time should then be screened for GDM at 24 to 28 weeks of gestation.
Screening for GDM is done using a 75-g 2-hour oral glucose tolerance test (2-h OGTT).
The American College of Obstetricians and Gynecologists (ACOG) issued a Committee statement asserting that they have not yet adopted the new diagnostic criteria for gestational diabetes (as listed on
page 944) and will continue to use the diagnostic criteria established by either Carpenter and Coustan or the National Diabetes Data Group. Both of these screening methods utilize a 100-g 3-h OGTT, require two abnormal results and the threshold for these results is higher than the newer criteria.
Diabetes Due to Other Causes
Genetic defects of insulin secretion and/or insulin action.
Pancreatic diseases (such as cystic fibrosis, pancreatitis, and pheochromocytoma).
Drug-induced (such as corticosteroids and medications used to treat HIV/AIDS).
Prediabetes
Prediabetes is an abnormality in glucose values intermediate between normal and overt diabetes.
Associated with obesity, dyslipidemia, and hypertension; prediabetes is a potent risk factor for developing T2DM and cardiovascular disease.
Every effort should be made to assist the patient with aggressive lifestyle modifications (diet, exercise, and weight loss) to prevent the progression from prediabetes to overt T2DM.
There are two forms of prediabetes, based on when glucose is elevated. Patients that simultaneously have both forms of prediabetes have a very high risk of developing T2DM.
Impaired fasting glucose (IFG)—defined as having fasting blood glucose levels of 100 to 125 mg/dL.
Impaired glucose tolerance (IGT)—defined as blood glucose measurement of 140 to 199 mg/dL using a 2-hour OGTT.
Laboratory Tests
American Diabetes Association (ADA). (2013). Standards of medical care in diabetes (position statement). Diabetes Care, 36(Suppl. 1), S11-S66.
Global International Diabetes Federation (IDF)/International Society of Pediatric and Adolescent Diabetes (ISPAD). (2011). Guideline for diabetes in childhood and adolescence. Available: www.ispad.org/NewsFiles/IDF-ISPAD_Diabetes_in_ Childhood_and%20Adolescene_Guidelines_2011.pdf.
Laboratory tests include those tests used to make the diagnosis as well as measures to monitor short- and long-term glucose control. See
Box 25-1 for details of when and how often to screen adults, adolescents, and children for T2DM.
Blood Glucose
Description
Fasting blood sugar (FBS), drawn after at least an 8-hour fast, to evaluate circulating amounts of glucose.
Postprandial test, drawn usually 2 hours after a well-balanced meal, to evaluate glucose metabolism.
Random glucose, drawn at any time, without regard to the time of last caloric intake.
Nursing and Patient Care Considerations
For fasting glucose, make sure that patient has maintained 8-hour fast overnight; sips of water are allowed.
Postprandial test, drawn usually 2 hours after a well-balanced sampling because this affects the test results.
For postprandial glucose, advise patient that no additional food or caloric beverages should be consumed during the 2-hour interval.
For random blood glucose, note the time and content of the last meal.
Diagnostic Criteria and Treatment Goals for Blood Glucose Values
American Diabetes Association (ADA) and American Association of Clinical Endocrinologists (AACE) diagnostic blood glucose values for diabetes mellitus in children, adolescents, and nonpregnant adults are:
FBS greater than or equal to 126 mg/dL, confirmed with a repeat test on another day.
Random blood sugar (regardless of time of last caloric intake) 200 mg/dL or higher and presence of classic symptoms of diabetes (polyuria, polydipsia, polyphagia, and weight loss). This test does not need to be repeated nor does the diagnosis need to be confirmed with another test.
Fasting blood glucose result of 100 to 125 mg/dL is prediabetes (IFG) and demands close follow-up and repeat monitoring at least every 3 years.
Blood glucose treatment goals for many nonpregnant adults with diabetes mellitus:
The International Diabetes Federation (IDF)/International Society of Pediatric and Adolescent Diabetes (ISPAD) blood glucose treatment goals for children and adolescents:
Premeal: 90 to 145 mg/dL.
Postmeal: 90 to 180 mg/dL.
Bedtime: 120 to 180 mg/dL.
ADA and AACE blood glucose treatment goals for women with gestational diabetes:
Premeal: 95 mg/dL or lower.
1 hour postmeal: 140 mg/dL or lower.
2 hours postmeal: 120 mg/dL or lower.
ADA and AACE blood glucose treatment goals for pregnant women with preexisting diabetes:
Oral Glucose Tolerance Test
Description
The oral glucose tolerance test (OGTT) evaluates insulin response to glucose loading. FBS is obtained before the ingestion of a glucose load and blood samples are drawn at timed intervals.
Nursing and Patient Care Considerations
Diagnostic Criteria Using the 75-g 2-h OGTT
ADA and AACE diagnostic OGTT values when screening for diabetes mellitus in children, adolescents, and nonpregnant adults:
ADA and AACE diagnostic blood glucose values for gestational diabetes performed 24 to 28 weeks gestation: (only need to meet the limits of one of the following values):
Fasting of 92 mg/dL or higher, or
180 mg/dL or higher at the 1-hour interval, or
153 mg/dL or higher at the 2-hour interval.
Glycated Hemoglobin (Glycohemoglobin, HbA1c, A1c)
Description
Measures glycemic control over a 60- to 120-day period by measuring the irreversible reaction of glucose to hemoglobin through freely permeable erythrocytes during their 120-day life cycle. Currently used to screen for diabetes as well as to monitor control.
Nursing and Patient Care Considerations
No prior preparation, such as fasting or withholding insulin/medications, is necessary.
Test results can be affected by red blood cell disorders (eg, thalassemia, sickle cell anemia), room temperature, ionic charges, and ambient blood glucose values.
Many methods exist for performing the test, making it necessary to consult the laboratory for normal values.
Should be performed at least twice yearly in patients whose diabetes is stable and well controlled. Quarterly (every 3 months) testing is recommended for patients who have had treatment changes/adjustments or whose diabetes is not well controlled.
Diagnostic Criteria and Treatment Goals for A1c Values
ADA and AACE diagnostic A1c values when screening for diabetes mellitus in children, adolescents, and nonpregnant adults:
6.5% or higher (should be confirmed with repeat A1c or fasting glucose or OGTT).
5.7% to 6.4% (ADA) or 5.5% to 6.4% (AACE) is considered prediabetes and demands close follow-up and repeat monitoring at least every 3 years.
The joint position statement (2009) of the ADA, the American College of Cardiology Foundation, and the American Heart Association asserts that in general, the A1c goal for nonpregnant adults is less than 7%, but that this must be individualized based on life expectancy, duration of diabetes, history of hypoglycemia, presence of microvascular complications, and presence of cardiovascular disease.
The IDF/ISPAD treatment A1c goal for children is between 7.5% and less than 9% based on age and ability to detect hypoglycemia.
The ADA and AACE treatment A1c goal of pregnant women with preexisting type 1 or type 2 diabetes is less than 6% (if without excessive hypoglycemia).
Because GDM is diagnosed late in pregnancy, the A1c is not a reliable marker of control (3-month average) and the fructosamine value (2- to 3-week average) may be more meaningful.
Fructosamine Assay
Description
Glycated protein with a much shorter half-life than glycated hemoglobin, reflecting control over a shorter period, approximately 14 to 21 days. May be advantageous in patients with hemoglobin variants that interfere with the accuracy of glycated hemoglobin tests.
Nursing and Patient Care Considerations
Note if patient has hypoalbuminemia or elevated globulins because test may not be reliable.
Should not be used as a diagnostic test for diabetes mellitus.
No special preparation or fasting is necessary.
C-Peptide Assay (Connecting Peptide Assay)
Description
Cleaved from the proinsulin molecule during its conversion to insulin, C-peptide acts as a marker for endogenous insulin production.
Nursing and Patient Care Considerations
Test can be performed after an overnight fast or after stimulation with Sustacal, intravenous (IV) glucose, or 1 mg of Glucagon SubQ.
Absence of C-peptide indicates no beta cell function, reflecting possible T1DM or insulinopenia in T2DM.
Autoantibody Testing
Description
An
autoantibody is an antibody (a type of protein) manufactured by the immune system that is directed against one or more of the
individual’s own proteins. Islet cell autoantibodies are strongly associated with the development of T1DM. The appearance of autoantibodies to one or several of the autoantigens—GAD65, IA-2, or insulin—signals an autoimmune pathogenesis of β-cell destruction. The positivity of any or all of these autoantibodies in the presence of hyperglycemia is used to confirm the diagnosis of T1DM. Because T1DM affects such a small number of the population, generalized screening would be costly and therefore is not recommended.
Nursing and Patient Care Considerations
 Evidence Base
Evidence Base Evidence Base
Evidence Base Evidence Base
Evidence Base NURSING ALERT
NURSING ALERT Evidence Base
Evidence Base Evidence Base
Evidence Base DRUG ALERT
DRUG ALERT




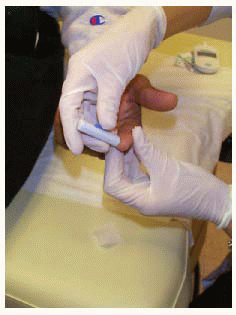 Obtaining blood from a finger using a lancet device
Obtaining blood from a finger using a lancet device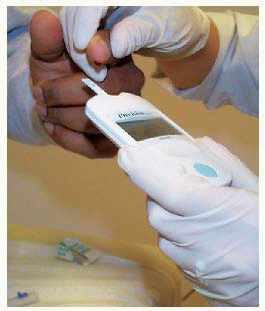 Applying drop of blood to test strip
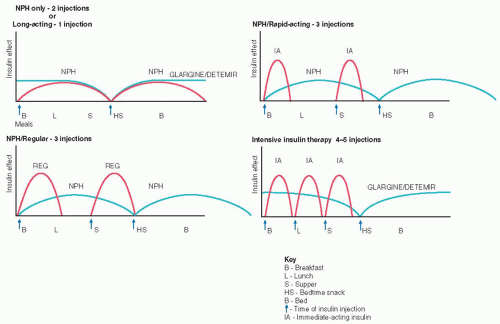
Applying drop of blood to test strip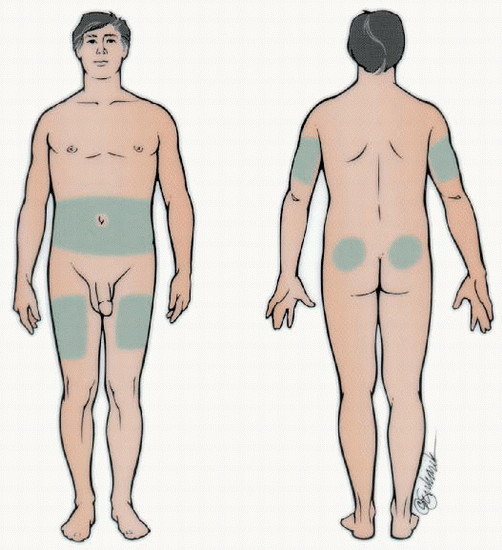 Rotate sites within each body part and use the same body part for the same injection time each day. Absorption is quicker from the abdomen and arms than the thighs and buttocks. Exercising a body part will hasten insulin absorption, so exercise should be consistent.
Rotate sites within each body part and use the same body part for the same injection time each day. Absorption is quicker from the abdomen and arms than the thighs and buttocks. Exercising a body part will hasten insulin absorption, so exercise should be consistent. DRUG ALERT Lantus and Levemir insulins must never be mixed with any other insulin in the same syringe.
DRUG ALERT Lantus and Levemir insulins must never be mixed with any other insulin in the same syringe. Evidence Base Frid, A., Hirsch, L., Gaspar, R., et al. (2010). New injection recommendations for patients with diabetes. Diabetes and Metabolism, 36(Suppl. 2), S3-S18.
Evidence Base Frid, A., Hirsch, L., Gaspar, R., et al. (2010). New injection recommendations for patients with diabetes. Diabetes and Metabolism, 36(Suppl. 2), S3-S18.