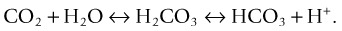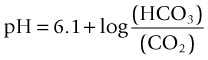13 Respiratory Assessment and Monitoring
After reading this chapter, you should be able to:
• demonstrate an understanding of respiratory anatomy and normal physiology
• describe the mechanisms that contribute to altered respiratory function
• examine the key principles underpinning assessment and monitoring of respiratory function
• discuss nursing assessment and monitoring activities for critically ill patients with respiratory dysfunction
• explain the importance of patient assessment skills, and the contribution of diagnostic and laboratory findings to ongoing clinical management
• justify the physiological bases for different types of monitoring
• discuss some common forms of diagnostic procedures used in critical care
Introduction
This chapter provides a comprehensive description of the principles and practice of respiratory assessment, monitoring, and diagnostics. This knowledge is important in providing timely and effective interventions for critically ill patients with respiratory dysfunction. The following two chapters then discuss the management of respiratory alterations (Chapter 14) and oxygenation and ventilation interventions (Chapter 15).
Related Anatomy and Physiology
The thorax cavity contains the trachea and bronchial tree, the two lungs, pleura and diaphragm. The mediastinum, located between the lungs, houses and protects the heart, great vessels and the oesophagus. Twelve pairs of ribs cover the lungs, ten of which are connected to the spine posteriorly, and to the sternum or to the cartilage of the rib above anteriorly (ribs 8–10). The 11th and 12th ribs have no anterior attachment (see Figure 13.1).1
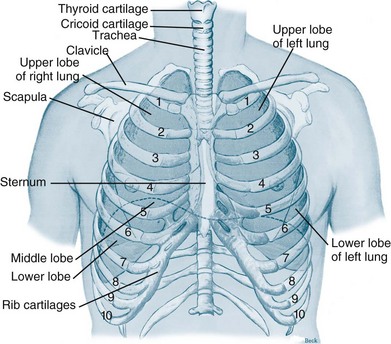
FIGURE 13.1 Ventilatory structures of the chest wall and lungs, showing the ribs and lobes of the lungs.1
The respiratory system is divided into upper and lower respiratory tracts: the upper airways consist of the nose, nasal conchae, sinus and pharynx; the lower respiratory tract includes the larynx, trachea, bronchi and lungs.2 Larger airways are lined with stratified epithelial tissue, which have a relatively high cellular turnover rate; these cells protect and clear these large airways. There are also additional specialised features of this tissue including an extensive distribution of mucus/goblet cells and cilia, which facilitate the mucociliary clearance system and aid airway clearance.
Upper Respiratory Tract
The nasal cavities contain an extremely vascular and mucoid environment for warming and humidifying inhaled gases. To maximise exposure to this surface area, the nasal conchae create turbulent gas flow. Cilia at the top of the epithelial cells and mucus provide filtration and cleaning of the inhaled air. Mucus is moved by the cilia lining the conducting airways towards the pharynx at a rate of 1–2 cm per minute. One litre of mucus is produced every day with only a small part not reabsorbed by the body.3,4
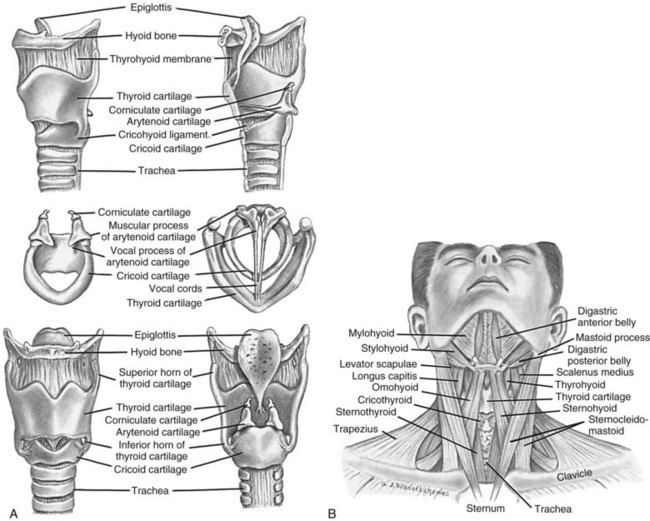
The pharynx is a muscular tube that transports food and air to the oesophagus and larynx, respectively. Inferior to the pharynx, the larynx consists mostly of cartilage attached to other cartilage and surrounding structures, and houses the vestibular (false) vocal folds and the true vocal cords (see Figure 13.2).5 An important pair of cartilages within the larynx is the pyramid-shaped arytenoids, which act as attachment points for the vocal cords. This area is easily damaged by pressure from endotracheal tubes; the most significant independent risk factor for injury to the arytenoids is the length of intubation time.6 The thyroid cartilage (‘Adam’s apple’) and the cricoid cartilage protect the glottis and the entrance to the trachea.4 Another cartilage in the larynx is the triangular-shaped elastic epiglottis which protects the lower airways from aspiration of food and fluids into the lungs. The epiglottis usually occludes the inlet to the larynx during swallowing. The primitive cough, swallow and gag reflexes further protect the airway.4
Lower Respiratory Tract
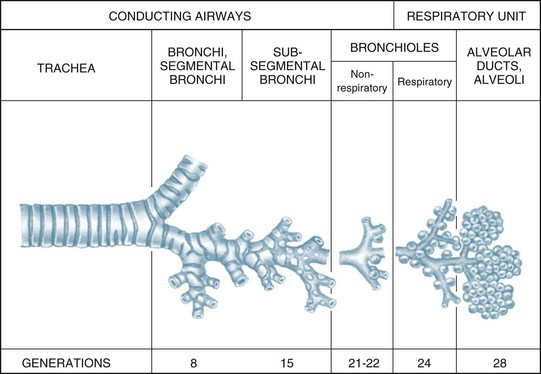
The airways within each lung branch out further into secondary (or lobar) bronchi then tertiary (or segmental) bronchi. Further divisions within these conducting airways end with the terminal bronchioles, the smallest airways without alveoli. These conducting airways do not participate in gas exchange but form the anatomical dead space (approximately 150 mL).7
Larger airways have a greater proportion of supporting cartilage, ciliated epithelium, goblet and serous cells and hence a mucous layer. As the airways become smaller, cartilage becomes irregularly dispersed, the number of goblet cells and amount of mucus decreases until, at the alveolar level, there is only a single layer of squamous epithelial cells. Alveolar macrophages are present in these epithelial cells, and phagocytose any small particles that may enter the alveolar area. Smooth muscle surrounds and supports the bronchioles, enabling airway diameter change and subsequent changes in airway resistance to gas flow.8
Thorax/Lungs
The lungs and heart are protected within the thoracic cage. Expansion of the thorax enables the lungs to fill with air during inspiration when respiration is triggered, and to passively compress to expel air from the lungs during expiration. The diaphragm separates the thorax from the abdomen and actively participates in the ventilation process. The diaphragm is the most important inspiratory muscle, performing approximately 80% of the work of breathing. Inspiration is initiated from the medulla, sending impulses through the phrenic nerve to stimulate the diaphragm to contract and flatten. The phrenic nerve originates in the cervical plexus and involves the third to fifth cervical nerves. It splits into two parts, passing to the left and right side of the heart before it reaches the diaphragm. For this reason, patients can have ventilation difficulties if phrenic nerve damage is due to C3–C5 trauma.8,9
The conducting airways move inspired air towards the respiratory unit, ending in the terminal bronchioles. The respiratory bronchioles, the alveolar ducts and alveolar sacs form the respiratory unit where the diffusion of gas molecules, or gas exchange, occurs. The respiratory unit makes up most of the lung with a volume of 2.5–3 L during rest7 (see Figure 13.3).
Surfactant
Of particular importance to the structure and function of the respiratory system are the type I and II alveolar epithelial cells. Type I cells provide support of the wall within the alveolar unit. Type II cells produce an important lipoprotein, surfactant, that lines the inner alveolar surface, and lowers surface tension of the alveoli, stabilising the alveoli to optimise lung compliance and facilitate expansion during inspiration.7 If surfactant synthesis is reduced due to pulmonary disease, lung compliance decreases and the work of breathing increases.10
Pleura
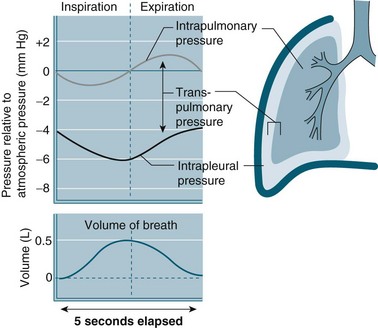
The intra-pleural pressure in the pleural space under normal circumstances is always negative with a range of −4 to −10 cmH2O; this negative pressure keeps the lungs inflated. During inhalation the pressure becomes more negative as both the lungs and the chest wall are elastic structures. These elastic fibres of the lung pull the visceral pleura inwards while the chest wall pulls the parietal pleura outward. The pressure difference between the alveolar pressure (0 cmH2O pressure in the lungs) and the intra-pleural pressure (−4 cmH2O) across the lung wall is termed the trans-pulmonary pressure (+4 cmH2O [0 − (−4) = +4]), and is the force that hold the lungs open3,4 (see Figure 13.4).
Pulmonary Circulation
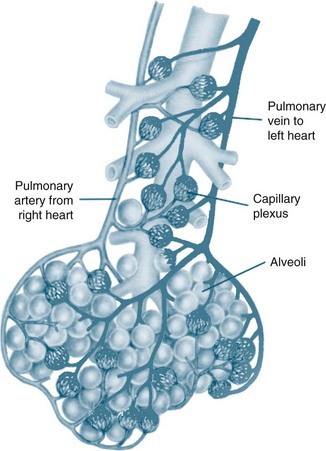
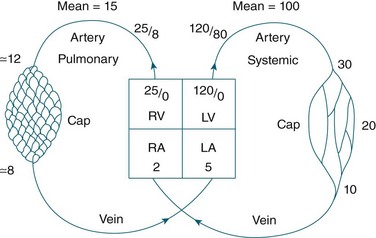
The circulatory system of the lung receives the entire cardiac output but operates as a low pressure system, as it only directs blood back to the left side of the heart (unlike the systemic circulation which pumps blood to different regions of the entire body). The pulmonary circulation involves oxygen-depleted blood being pumped by the right ventricle to the lungs via the pulmonary artery, with oxygen-rich blood returning to the left atrium via the pulmonary veins. Pulmonary blood vessels follow the path of the bronchioles, with the capillaries forming a dense network in the walls of the alveoli. As illustrated in Figure 13.5,5 the entire surface area of the alveolar wall is covered by these capillaries, where gas exchange occurs as the capillaries are just large enough for a red blood cell to pass through.
Pulmonary vessels are short, thin and have relatively little smooth muscle. The pressure inside the vessels is remarkably low (normal pulmonary artery pressure is only 25/8 mmHg; mean 15 mmHg).7 This low pressure system ensures that the work of the right heart is as small as feasible, while promoting efficient gas exchange in the lungs11 (see Figure 13.6).
Bronchial Circulation
The bronchial circulation, part of the systemic circulation, supplies oxygenated blood, nutrients and heat to the conducting airways (to the level of the terminal bronchioles) and to the pleura. Drainage of this deoxygenated blood is predominantly through the bronchial network, although some capillaries drain into the pulmonary arterial circulation, contributing to venous admixture or right-to-left shunt7 (see Pathophysiology below for further discussion).
Control of Ventilation
Controller
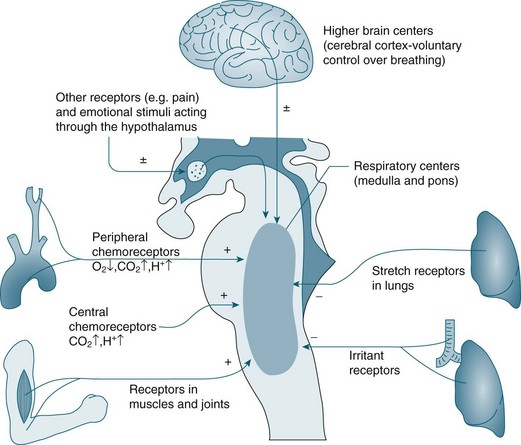
In the brainstem, the medulla oblongata and the pons regulate automatic ventilation while the cerebral cortex regulates voluntary ventilation (see Figure 13.7). The respiratory rhythmic centre in the medulla can be divided into inspiratory and expiratory centres, with the following functions:8
• The inspiratory centre (or dorsal respiratory group) triggers inspiration.
• The expiratory centre (or ventral respiratory group) only functions during forced respiration and active expiration.
• The pneumotaxic and apneustic centre in the pons adjusts the rate and pattern of breathing.
• The cerebral cortex provides conscious voluntary control over the respiratory muscles. This voluntary control cannot be maintained when PCO2 and hydrogen ion (H+) concentration become markedly elevated; an example is the inability to hold your breath for very long.8 Emotional and autonomic activities also often affect the pace and depth of breathing.
Effectors
The diaphragm is the major muscle of inspiration, although the external intercostal muscles are also involved. The accessory muscles of inspiration (scalenes, sternocleidomasteoid muscles and the pectoralis minor of the thorax) are active only during exercise or strenuous breathing. Expiration is a passive act and only the internal intercostal muscles are involved at rest. During exercise, the abdominal muscles also contribute to expiration.4 Inspiration is triggered by stimulus from the medulla, causing the diaphragm to contract downwards, and the external intercostal muscles to contract, lifting the thorax up and out. This action lowers pressure within the alveoli (intra-alveolar pressure) relative to atmospheric pressure. Air rushes into the lungs to equalise the pressure gradient. After contraction has ceased, the ribs and diaphragm relax, the pressure gradient reverses, and air is passively expelled from the lungs and return to their resting state due to elastic recoil.
Sensors
A chemoreceptor is a sensor that responds to a change in the chemical composition of the blood; there are two types: central and peripheral. Central chemoreceptors account for 70% of the feedback controlling ventilation, and respond quickly to changes in the pH of cerebral spinal fluid (CSF) (increase of PCO2 in arterial blood).9 If the PCO2 in arterial blood remains high for a prolonged period, as in chronic obstructive pulmonary disease (COPD), a compensatory change in HCO3 occurs and the pH in CSF returns to its near normal value.7 Under these conditions a patient breathes due to hypoxic drive; that is, low levels of O2 are detected by peripheral chemoreceptors and this triggers breathing. For this small percentage of the population with COPD, care is required when administering oxygen so that the stimulus to breathe is not compromised, as increases in PO2 may reduce respiratory drive. Peripheral chemoreceptors respond to low partial pressure of oxygen in arterial blood (PaO2) and contribute to maintaining ventilation, functioning optimally when oxygen levels fall below 70 mmHg.7
Central chemoreceptors located in the medulla respond to changes in hydrogen ion concentration in the CSF that surrounds these receptors. A change in the partial pressure of carbon dioxide in arterial blood (PaCO2) causes movement of CO2 across the blood–brain barrier into the CSF and alters the hydrogen ion concentration. This increase in hydrogen ions stimulates ventilation. Central chemoreceptors do not however respond to changes in PaO2 levels. Opiates also have a negative influence on these chemoreceptors causing less sensitivity to changes in hydrogen ion concentration.7 Note also that hyperventilation may reduce the level of PaCO2 to a level that could cause accidental unconsciousness if the breath is held after hyperventilation. This phenomenon is well known amongst divers and is due to increasing levels of CO2 as the primary trigger of breathing. If the CO2 level is too low due to hyperventilation, the breathing reflex is not triggered until the level of oxygen has dropped below what is necessary to maintain consciousness.
Peripheral chemoreceptors are located in the common carotid arteries and in the arch of the aorta. These receptors are sensitive to changes in PaO2 and are the primary responders to hypoxaemia, stimulating the glossypharyngeal and vagus nerves and providing feedback to the medulla. Peripheral chemoreceptors also detect changes in PaCO2 and hydrogen ion concentration/pH in arterial blood.9 Other receptors include stretch receptors located in the lungs that inhibit inspiration and protect the lungs from over-inflation (Hering–Breuer reflex), and in the muscles and joints (see Figure 13.7).
Pulmonary Volumes and Capacities
In healthy individuals, the lungs are readily distensible or compliant; when exposed to high expanding pressures or in disease states, compliance is increased or decreased. A range of lung volumes and capacities are illustrated in Figure 13.8. Tidal volume (TV) is the volume of air entering the lungs during a single inspiration and is normally equal to the volume leaving the lungs on expiration (around 500 mL). During inspiration, the TV of inspired air is added to the 2400 mL of air already in the lungs. This volume of air that remains in the lungs after a normal expiration is the functional residual capacity (FRC),4 which:
• has an important role in keeping small alveoli open and avoiding atelectasis
• can be reduced during anaesthesia or neuromuscular blockade, most likely due to loss of muscle tone12
• if reduced, results in the smallest alveoli closing at the end of the expiration (the ‘closing volume’).
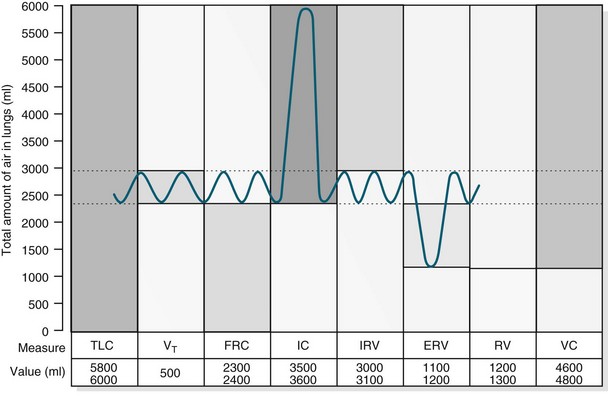
FIGURE 13.8 For lung volume measurements, all values are approximately 25% less in women. ERV, expiratory reserve volume; IC, inspiratory capacity; IRV, inspiratory reserve volume; FRC, functional residual capacity; TLC, total lung capacity; RV, residual volume; VC, vital capacity; VT, tidal volume.10
The closing volume plus the residual volume is called the ‘closing capacity’. The closure of the smallest airways may occur because dependent areas of the lungs are compressed, although this is not the only mechanism as these airways also close in the weightlessness of space. The closing volume is dependent on patient age; in a young healthy person it is 10% of vital capacity, while for an individual aged 65 years it increases to 40%, approximating total FRC.11
Alveolar Ventilation
Minute volume (MV), often referred to during mechanical ventilation, is TV multiplied by respiratory frequency (e.g. 500 mL × 12 breaths per minute = 6000 mL MV). Importantly, only the first 350 mL of inhaled air in each breath reaches the alveolar exchange surface, with 150 mL remaining in the conducting airways (called the ‘anatomic dead space’). Alveolar ventilation is the amount of inhaled air that reaches the alveoli each minute (e.g. 350 mL × 12 = 4200 mL of alveolar ventilation).8
Work of Breathing
In a resting state, energy requirements to breathe is minimal (less than 5% of total O2 consumption).7 However, changes in airway resistance and lung compliance affect the work of breathing (WOB), resulting in increased oxygen consumption (VO2).13 As noted earlier, the lungs are very distensible and expand during inspiration. This expansion is called the elastic or compliance work and refers to the ease by which lungs expand under pressure. Lung compliance is often monitored when patients are mechanically ventilated, and is calculated by dividing the change in lung volume by the change in trans-pulmonary pressure.3 For the lung to expand, it must overcome lung viscosity and chest wall tissue (called ‘tissue resistance work’). Finally, there is airway resistance work – movement of air into the lungs via the airways. The work associated with resistance and compliance is easily overcome in healthy individuals but in pulmonary disease, both resistance and compliance work is increased.3,14 During exertion, when increased muscle function heightens metabolic rate, oxygen demand rises to match consumption and avoid anaerobic metabolism, and work of breathing is increased. The term ‘work of breathing’ is often used in those who are critically ill, when basic respiratory processes are challenged and breathing consumes a far greater proportion of total energy.
Principles of Gas Transport and Exchange in Alveoli and Tissues
Oxygen and carbon dioxide is transported in the bloodstream between the alveoli and the tissue cells by the cardiac output. Delivery of oxygen to tissues and transfer of carbon dioxide from the tissues to the capillary occurs by diffusion and is therefore dependent on the pressure gradient between the capillary and the cell. Diffusion involves molecules moving from areas of high concentration to low concentration. Other determinants of the rate of diffusion include the thickness of the alveolar membrane, the amount of surface area of the membrane available for gas transfer and the inherent solubility of the gas. Carbon dioxide diffuses about 20 times more rapidly than oxygen because of the much higher solubility of carbon dioxide in blood.7 At the most distal ends of the conducting airways lies an extensive network of approximately 300 million alveoli. The surface area of the lungs if spread out flat is about 90 m2 – about 40 times greater than the surface of the skin.4 Gas exchange occurs through the exceptionally thin alveolar membranes. Oxygen uptake takes place from the external environment via the lungs through to the blood in the adjacent alveolar capillary networks. Similarly, carbon dioxide diffuses from capillaries to the alveoli and is then expired.
Oxygen Transport
In oxygenated blood transported by the pulmonary capillaries, there is 20 mL of oxygen in each 100 mL of blood. Oxygen is transported in two ways; dissolved in plasma (about 0.3 mL; 1.5%) with the remainder bound to haemoglobin.8 The 1.5% of oxygen dissolved in the blood is what constitutes PaO2 and measured by arterial blood gases.4 One gram of haemoglobin carries 1.34 mL oxygen, and the level of saturation within the total circulating haemoglobin can be measured clinically, commonly by pulse oximetry. The amount of oxygen actually bound to haemoglobin compared with the amount of oxygen the haemoglobin can carry is commonly reported as SaO2. Oxygen is attached to the haemoglobin molecule at four haem sites. As the majority of oxygen transport is via haemoglobin, if all four sites are occupied with oxygen molecules the blood is determined to be ‘fully saturated’ (SaO2 = 100%).14
A large reserve of oxygen is available if required, without the need for any increase in respiratory or cardiac workload. Oxygen extraction is the percentage of oxygen extracted and utilised by the tissues. At rest, just 25% of the total oxygen delivered to the tissue is extracted, although this amount does vary throughout the body, with some tissue beds extracting more and others taking less. Normally, the oxygen saturation of venous blood is 60–75%; values below this indicate that more oxygen than normal is being extracted by tissues. This can be due to a reduction in oxygen delivery to the tissues, or to an increase in the tissue consumption of oxygen.8,9
Oxygen delivery (DO2) and oxygen consumption (VO2) are important aspects to consider in the management of a critically ill patient. Normal oxygen delivery in a healthy person at rest is approximately 1000 mL/min. Normal oxygen consumption is 200–250 mL/min,9 but this can increase significantly during episodes of sepsis, fever, hypercatabolism and shivering.14 The difference between normal delivery and normal consumption highlights the large degree of oxygen reserve available to the body.
Oxygen–Haemoglobin Dissociation Curve
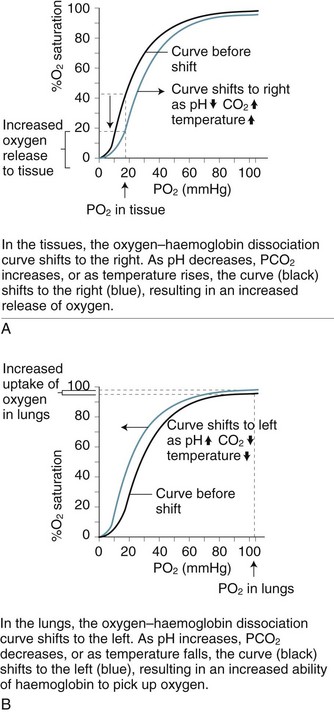
As blood is transported to the tissues and end-organs, the affinity of haemoglobin and oxygen to combine decreases, relative to the surrounding arterial oxygen tension. This relationship is illustrated by the oxyhaemoglobin dissociation curve (see Figure 13.9). As oxygen is offloaded at the tissue level, carbon dioxide binds more readily with haemoglobin, to be transported back to the lungs for removal.4
In the upper part of the curve (within the lungs), relatively large changes in the PaO2 cause only small changes in haemoglobin saturation. Therefore, if the PaO2 drops from 100 to 60 mmHg (14–8 kPa), the saturation of haemoglobin changes only 7% (from a normal 97% to 90%). The lower portion (steep component) of the oxygen–haemoglobin dissociation curve, when PaO2 is between 60 and 40 mmHg (8–5 kPa) reflects however that as haemoglobin is further de-saturated, larger amounts of oxygen are released for tissue use, ensuring an adequate oxygen supply to peripheral tissues is maintained even when oxygen delivery is reduced.4 Oxygen saturation still remains at 70–75%, leaving a significant amount of oxygen in reserve. The relationship between the two axes of this curve assumes normal values for haemoglobin, pH, temperature, PaCO2 and 2,3-DPG. Changes to any of these values will shift the curve to the right or left and therefore reflect different values for PaO2 and SaO2.8
Carbon Dioxide Transport
Carbon dioxide is transported by blood in three forms: combined with water as carbonic acid (80–90%), dissolved (5%), or attached to plasma proteins (5–10%), including haemoglobin. The dissolved carbon dioxide constitutes PaCO2 and is measured by arterial blood gases. The greater solubility of CO2 when compared with oxygen results in rapid diffusion across the capillary membranes, and therefore the gas can be easily removed for elimination.4 Carbon dioxide, a byproduct of cellular respiration, is produced at a rate of 200 mL/min, with only minor differences in normal concentrations in arterial (480 mL/L) and venous (520 mL/L) blood.9
Relationship Between Ventilation and Perfusion
Gas exchange is the key function of the lungs, and the unique anatomy of capillaries and alveoli facilitates this process. However, a number of physiological factors mean that the ventilation (V) to perfusion (Q) ratio is not matched in a 1 : 1 relationship. As normal alveolar ventilation is about 4 L/min and pulmonary capillary perfusion is about 5 L/min, the normal ventilation to perfusion ratio (V/Q) is 0.8.7 In addition, pressure in the pulmonary circulation is low relative to systemic pressure, and is influenced much more by gravity/hydrostatic pressure. In the upright position, lung apices receive less perfusion compared with the bases.7 In the supine position, apical and basal perfusion is almost equal, but the posterior (dependent) portion of the lungs receives greater perfusion than the anterior lung area. Ventilation is also uneven throughout the lung, with the bases receiving more ventilation per unit volume than the apices.7
For a patient in an upright position, in:
• Zone 1 (upper area of the lungs): alveolar pressure is generally greater than both arterial and venous capillary pressure [PA>Pa>Pv], and blood flow is reduced, leading to alveolar dead space (alveoli ventilated but not adequately perfused).
• Zone 2 (middle portion of the lungs): perfusion and gas exchange is influenced more by pressure differences between arterial and alveolar pressures than by the usual difference between arterial and venous pressures [Pa>PA>Pv], with a normal V/Q ratio.
• Zone 3 (lung bases): alveolar pressure is lower than both arterial and venous pressures [Pa>Pv>PA], and ventilation is reduced leading to intrapulmonary shunting (alveoli perfused but not adequately ventilated)7 (see Figure 13.10).
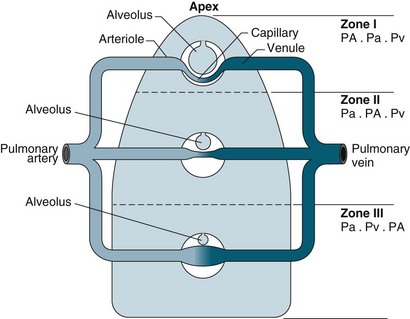
FIGURE 13.10 The effects of gravity and alveolar pressure on pulmonary blood flow. Notice the three lung zones.86
These physiological relationships are more complex in a critically ill patient when ventilation and/or lung perfusion is further compromised by disease processes and positive pressure ventilation, and the patient is in a supine or semi-recumbent position.7
Acid–Base Control: Respiratory Mechanisms
(6.1 = the dissociation constant in plasma).11
Respiratory alkalosis occurs when a patient hyperventilates with large, frequent breaths; CO2 decreases in arterial blood and pH rises. If this condition is maintained (e.g. walking at high altitude), the kidney excretes bicarbonate and pH returns to normal (i.e. the respiratory alkalosis is compensated).11
Pathophysiology
Three common pathophysiological concepts that influence respiratory function in critically ill patients are hypoxaemia, inflammation and oedema. The principles for these phenomena are discussed below. Related presenting disease states including respiratory failure, pneumonia, acute lung injury, asthma and chronic obstructive pulmonary disease are described in Chapter 14.
Hypoxaemia
Hypoxaemia describes a decrease in the partial pressure of oxygen in arterial blood (PaO2) of less than 60 mmHg.4 This state leads to less efficient anaerobic metabolism at the tissue and end-organ level, and resulting compromised cellular function. Hypoxia is abnormally low PO2 in the tissues, and can be due to:
• ‘hypoxic’ hypoxia: low PaO2 in arterial blood due to pulmonary disease
• ‘circulatory’ hypoxia: reduction of tissue blood flow due to shock or local obstruction
• ‘anaemic’ hypoxia: reduced ability of the blood to carry oxygen due to anaemia or carbon monoxide poisoning
• ‘histotoxic’ hypoxia: a cellular environment that does not support oxygen utilisation due to tissue poisoning (e.g. cyanide poisoning).7
A hypoxic patient can show symptoms of fatigue and shortness of breath if the hypoxia has developed gradually. If the patient has severe hypoxia with rapid onset, they will have ashen skin and blue discolouration (cyanosis) of the oral mucosa, lips, and nail beds. Confusion, disorientation and anxiety are other symptoms. In later stages, unconsciousness, coma and death occur.15
Acute respiratory failure is a common patient presentation in ICU that is characterised by decreased gas exchange with resultant hypoxaemia.16 Two different mechanisms cause acute respiratory failure: Type I presents with low PO2 and normal PCO2; Type II presents with low PO2 and high PCO21 (see Chapter 14 for further discussion).
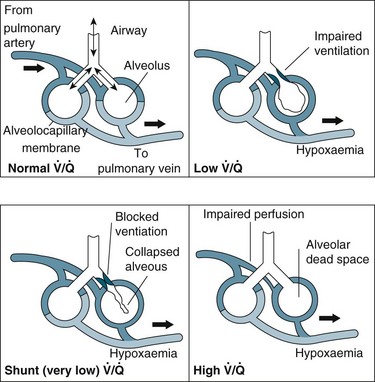
In general, impaired gas exchange results from alveolar hypoventilation, ventilation/perfusion mismatching and intrapulmonary shunting, each resulting in hypoxaemia. Hypercapnia may also be present depending on the underlying pathophysiology.17
Alveolar hypoventilation occurs when the metabolic needs of the body are not met by the amount of oxygen in the alveoli. Hypoxaemia due to alveolar hypoventilation is usually extrapulmonary (e.g. altered metabolism, interruption to neuromuscular control of breathing/ventilation) and associated with hypercapnia.17
Ventilation/perfusion (V/Q) mismatch results when areas of lung that are perfused are not ventilated (no participation in gas exchange) because alveoli are collapsed or infiltrated with fluid from inflammation or infection (e.g. pulmonary oedema, pneumonia). This results in an overall reduction in blood oxygen levels, which can usually be countered by compensatory mechanisms.1
Intrapulmonary shunting is an extreme case of V/Q mismatch. Shunting occurs when blood passes alveoli that are not ventilated. There can be significant intrapulmonary shunting, and therefore overwhelming reductions in PaO2.18 Carbon dioxide levels may still be normal but depending on the onset and progression of the respiratory pathophysiology, compensatory mechanisms may not be able to maintain homeostasis1,11 (see Figure 13.11).
Tissue Hypoxia
There are few physiological changes with mild hypoxaemia (when O2 saturation remains at 90% despite a PaO2 of 60 mmHg [8 kPa]), with only a slight impairment in mental state. If hypoxaemia deteriorates and the PaO2 drops to 40–50 mmHg (5.3–6.7 kPa), severe hypoxia of the tissues ensues. Hypoxia at the central nervous system level manifests with headaches and somnolence. Compensatory mechanisms include catecholamine release, and a decrease in renal function results in sodium retention and proteinuria.19
Different tissues vary in their vulnerability to hypoxia, with the central nervous system and myocardium at most risk. Hypoxia in the cerebral cortex results in a loss of function within 4–6 seconds, loss of conscious in 10–20 seconds and irreversible damage in 3–5 minutes.11 In an environment that lacks oxygen, cells function by anaerobic metabolism and produce much less energy (adenosine triphosphate [ATP]) than with aerobic metabolism (2 versus 38 ATP molecules per glucose molecule), and lactic acid increases. With less available energy, the efficiency of cellular functions such as the Na+/K+ pump, nerve conduction, enzyme activity and transmembrane receptor function diminishes.19 The overall effect of interruption to these vital cellular activities is a reduction in organ or tissue function, which in turn compromises system and body functions.
Changes to the oxyhaemoglobin dissociation curve also occur in states related to hypoxia. The curve shifts to the right when there is acidosis and/or raised levels of PCO2 as commonly seen in respiratory failure. Although this change may alter patient oxygen saturation readings, the increased release of oxygen from haemoglobin to the tissues has obvious benefits for tissue oxygenation and cellular metabolism.7
Compensatory Mechanisms to Optimise Oxygenation
When PO2 in the alveolus is reduced, hypoxic pulmonary vasoconstriction occurs, with contraction of smooth muscles in the small arterioles in the hypoxic region, directing blood flow away from the hypoxic area of the lung.7 Peripheral chemoreceptors also detect hypoxaemia and initiate compensatory mechanisms to optimise cellular oxygen delivery. Initial responses are increased respiratory rate and depth of breathing, resulting in increased minute ventilation, and raised heart rate with possible vasoconstriction as the body attempts to maintain oxygen delivery and uptake. This overall up-regulation cannot be sustained indefinitely, particularly in a person who is critically ill, and compensatory mechanisms begin to fail with worsening hypoxaemia and cellular and organ dysfunction. Unless the hypoxaemia is reversed and/or respiratory and cardiovascular support is provided, irreversible hypoxia and death will ensue.
Inflammation
Inflammatory processes can occur at a local level (e.g. as a result of inhalation injuries, aspiration or respiratory infections) or are secondary to systemic events (e.g. sepsis, trauma). Damage to the pulmonary endothelium and type I alveolar cells appear to play a key role in the inflammatory processes associated with ALI.20 Once triggered, inflammation results in platelet aggregation and complement release. Platelet aggregation attracts neutrophils, which release inflammatory mediators (e.g. proteolytic enzymes, oxygen free radicals, leukotrienes, prostaglandins, platelet-activating factor [PAF]). Neutrophils also appear to play a key role in the perpetuation of ALI/ARDS.1 As well as altering pulmonary capillary permeability, resulting in haemorrhage and fluid leak into the pulmonary interstitium and alveoli, mediators released by neutrophils and some macrophages precipitate pulmonary vasoconstriction. Resulting pulmonary hypertension leads to diminished perfusion to some lung areas, with dramatic alterations to both perfusion and ventilation leading to significant V/Q mismatches, and the subsequent signs and symptoms typically seen in patients with pulmonary inflammation/oedema.
Oedema
Pulmonary oedema also alters gas exchange, and results from abnormal accumulation of extravascular fluid in the lung. The two main reasons for this are: ‘increased pressure’ oedema, where there is an increase in hydrostatic or osmotic forces (e.g. left heart ventricular dysfunction or volume overload); and ‘increased permeability’ oedema, that results from increased membrane permeability of the epithelium or endothelium in the lung, allowing accumulation of fluid (also called ‘non-cardiogenic’). Resulting clinical syndromes are acute lung injury (ALI) or acute respiratory distress (ARDS) (see Chapter 14 for further discussion).
Changes to Respiratory Function
During the early exudative phase of ALI/ARDS, tachypnoea, signs of hypoxaemia (apprehension, restlessness) and an increase in the use of accessory muscles are usually evident as a result of infiltration of fluids into the alveoli. With impaired production of surfactant during the proliferative phase, respiratory function deteriorates, and dyspnoea, agitation, fatigue and the emergence of fine crackles on auscultation are common.1,11 Airway resistance is increased when oedema affects larger airways. Lung compliance is reduced as interstitial oedema interferes with the elastic properties of the lungs, and patients may be quite a challenge to adequately ventilate. Infiltration of type II alveolar cells into the epithelium may lead to interstitial fibrosis on healing,21causing chronic lung dysfunction.
Respiratory Dysfunction: Changes to Work of Breathing
If respiratory compromise is not reversed, there will be significant increases to the work of breathing. Clinical manifestations include tachypnoea, tachycardia, dyspnoea, low tidal volumes and diaphoresis. Hypercapnia will ensue, which further compromises respiratory muscle function and precipitates diaphragmatic fatigue. Oxygen consumption during breathing can be so great that reserve capacity is reduced. If patients with preexisting COPD (who may breathe close to the fatigue work level) experience an acute exacerbation, this can easily tip them into a fatigued state. Early identification and management of respiratory compromise before these stages improves patient outcomes.19