Chapter 12. Research Governance and Research Ethics
Carol Haigh
▪ Introduction
▪ Why have governance guidelines developed?
▪ Fundamentals of research governance
▪ Fundamentals of research ethics
▪ Example 1 – vulnerable groups
▪ Example 2 – internet research
▪ Conclusion
Why have governance guidelines developed?
The earlier development of research governance guidelines was driven by specific failures in research supervision systems which resulted in public scandals. In the USA a review of the systems available for protecting the public participants of research trials was initiated by the (unrelated) deaths of Jesse Gelsinger in 1999 and Ellen Roche in 2002. In the UK the Alder Hey organ retention scandal which was beginning to unfold in 1999 likewise prompted a review of the systems that were in place to ensure the safety of research volunteers. The results, from both sides of the Atlantic, highlighted serious and widespread deficiencies (Steinbrook 2002). This led the US and UK governments to examine the research culture more closely and to develop frameworks and guidelines that addressed more issues than the traditional ethical review. Other countries were quick to see the implications of such an approach and began to consider the concept of research governance with the same degree of urgency they had once applied to the concepts of ethical inquiry. The approach to research governance is slightly different across the world, with some countries such as Australia (NHMRC 2004) and the OECD countries (2003) taking an integrated approach to ethics and research governance while others, notably the UK (2005), prefer to set out researcher obligations to governance in a separate document designed to complement existing ethics guidance. Nevertheless, the fundamental principles are the same. Since the UK has articulated these governance principles most clearly, it is appropriate to consider the elements of good research governance within the UK definitions and framework.
Fundamentals of research governance
Research governance is a framework through which institutions are accountable for the ethical quality, scientific acceptability and management of participant safety in the research that they sponsor or permit. Although there is a variety of approaches to the application of good governance strategies, fundamental elements can be expressed as falling within one of five domains (Fig. 12.1). These are:
▪ ethics;
▪ science;
▪ information;
▪ health and safety and employment;
▪ financial and intellectual property (Department of Health 2001).
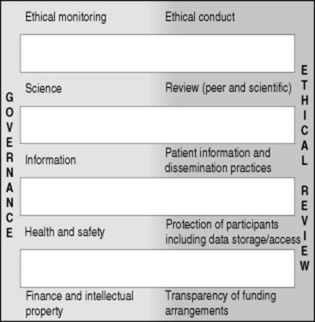 |
| Figure 12.1 How research governance and ethical review link together. |
Ethics
In the UK, ethics and governance are closely intertwined. Historically, once ethical approval had been obtained from the appropriate committee there was no monitoring of the subsequent research study, which rendered the system open to abuse. Governance processes are designed to promote monitoring of all aspects of research studies, in particular methods of obtaining consent, data protection and data storage. In the UK, governance departments must review 10% of all studies they approve each year.
Science
This governance domain is concerned with ensuring that the research that is carried out in the health and social care arena is scientifically robust, subject to expert review in its early stages and based as far as is practicable upon existing sources of information. This ensures that research is seen as a linear process with new work building upon that which has gone before. It requires potential researchers to show a thorough examination of the literature when submitting proposals for both ethical review and governance approval.
Information
Research that is not disseminated in the public domain is ‘dead’ research. Nonetheless a significant amount of research projects are completed without the results being made available to the interested parties. This is a particular concern with postgraduate research (Teacher Training Agency 1998). Even more projects are lodged in the public domain but are only accessible to a highly specialised or technical audience. Many grant-making bodies are now insisting that researchers should provide information for both the professional and the lay readership (Joseph Rowntree Foundation 2005). The governance province of ‘information’ helps to address the issue of research which is wasted through failure to disseminate, which in turn leads to failure to implement research findings. In an effort to ensure that research findings are implemented in health care practice, all proposed research projects must have considered how the anticipated findings will be disseminated across all interested parties. In the UK, the National Research Register records all NHS Trust research activities. This provides the public with information about ongoing and completed research projects.
Health and safety
This area of governance can be seen to address two significant areas. Firstly, it is connected to protection of participant well-being. Informed by the EU Clinical Trials Directive (2001/20/EU), published in May 2001, governance became part of UK legislation in 2003, and was fully implemented by 2004; the health and safety domain of governance frameworks requires that researchers have the protection and safety of participants in mind at all times. Prior to the development of governance frameworks and, for European countries, the drafting of the EU clinical trials directive (European Commission 2001), researchers were not obliged to report or disclose the health and safety issues inherent in their research. Additionally, in the UK, researchers who are not NHS employees, but who are carrying out research on NHS user/employee populations are required to obtain an honorary contract from the participating trust prior to commencing data collection. Concerns have been mooted that this often protracted process has the overall effect of, at best, slowing down research and, at worst, acting as a disincentive to health service research (Howarth & Kneafsey 2004). However, it does act as an additional protection for participants since it allows NHS establishments to satisfy themselves about the credibility and ability of the researcher who wishes to undertake research within their organisation.
The second area of health and safety concern addressed by this aspect of governance is focused upon the safety of the researchers themselves. This may mean assessing whether the researcher is likely to be exposed to ionising radiation or noxious substances during the course of the research study (not usually an issue for the majority of health and social care research studies for which nurses are likely to be responsible) or, more importantly, may concentrate upon issues such as visiting research participants in their own homes, or late night working. For the first time, researchers are encouraged to think about their personal safety.
Finance
Organisations involved in research activity must be able to demonstrate that research funds can be managed appropriately in line with any financial legislation. This includes compliance with audit requirements and the ability to compensate research participants in the event of negligent harm. In the UK, there is no strategy for the provision of compensation in the event of non-negligent harm. However, if the researcher is employed by an organisation which does provide indemnity against non-negligent harm, the organisation must also be able to demonstrate that they can offer that compensation.
Other considerations within the ‘finance’ governance domain overlap with the domain of intellectual property (see below) in that arrangements must be in place to protect and exploit any commercial benefit that may accrue from research studies.
Intellectual property
The UK, and the NHS in particular, has a poor record in terms of the exploitation of potential commercial benefits and opportunities that can be developed from research carried out within health care settings compared with other countries and organisations. The research governance framework is designed to ensure that the NHS benefits from new technologies and patentable materials that are generated by research within its environs by setting up monitoring processes and strategies for the protection of intellectual property rights.
Quality research culture
The creation of a quality research culture can be seen to have direct links to the research ‘incidents’ that drove the development of governance procedures. The UK government describe such a research culture as having the following characteristics:
▪ respect for participants’ dignity, rights, safety and well-being;
▪ valuing the diversity within society;
▪ personal and scientific integrity;
▪ leadership;
▪ honesty;
▪ accountability;
▪ openness;
▪ clear and supportive management (Department of Health 2001).
This aspect of research governance focuses upon clear identification of responsibility amongst the research team. The UK model proposes that strong research leadership should be allied to the promotion and support of research studies that reflect the national research agenda and the local priorities of the trust. Research governance frameworks set out definitions of the various roles within the research process such as:
▪ Principal investigator: the person responsible for the day-to-day management of the study who, by definition, has the skills and expertise to plan, direct and report the project.
▪ Sponsor: usually a representative of either the trust or the researcher’s employing organisation. Their role is to guarantee the quality of the research environment and the competence of the research team.
Responsibility for the safe, robust conduct of the research lies with the principal investigator; however, the investigator’s employing institution is also responsible for ensuring that the appropriate quality monitoring mechanisms are in place. Thus, the intention of research governance strategies is to improve research quality and safeguard the public by giving due consideration to all aspects of research project management via these five domains. Inherent to this intention are a number of prerequisites which are key conditions if the twin goals of improvement and protection are to be achieved. These are outlined in Table 12.1.
| Prerequisite | Governance domain |
|---|---|
| Enhancement of ethical and scientific quality | ▪ Ethics ▪ Science ▪ Financial and intellectual property |
| Reduction of adverse incidents and ensuring lessons are learned | ▪ Health, safety and employment ▪ Information |
| Prevention of poor performance and misconduct | ▪ Health, safety and employment |
These prerequisites form the basis of most of the approaches that have been taken to the management of research governance. For example, the European Forum for Good Clinical Practice overtly incorporated them into its recommendations and guidelines for European ethics committees (EFGCP 1997) whilst the OECD (2003) have taken a broader approach, using these concepts less explicitly as a framework for recommendations. The National Health and Medical Research Council of Australia (NHMRC 2004) have, in a draft document, produced governance guidelines that closely mirror those of the UK but which are far more directive.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
