Note about terminology
Although the terms are often used interchangeably, the term ‘oocyte’ refers to the developing gamete within the ovary, whereas the term ‘ovum’ (plural ova) refers to the gamete after ovulation had occurred. Oogenesis refers to the process of formation (mitotic division) of oocytes from oogonia (formed from the primordial germ cells). The primary oocyte is an oocyte which has undergone mitosis and entered meiosis (which is arrested in the 7th month of gestation). By the time the female infant is born, all of her oogonia are primary oocytes in meiotic arrest. The secondary oocyte is formed (together with the first polar body) when the first meiotic division is completed just before ovulation. So the ovum is the egg after the second meiotic division, when the second polar body is extruded. As fertilization triggers the completion of the second meiotic division, the newly-ovulated female gamete does not stay an ‘ovum’ very long as the sperm has already penetrated the ovum; once the chromosomes from the egg and sperm are united, the fertilized ovum is correctly known as an embryo. The one-celled embryo is also referred to as a zygote. At the 16-cell stage, the embryo is called a morula (Latin for mulberry). When the fluid-filled cavity (blastocoele) forms in the embryo separating the trophoblast cells from the inner cell mass, the embryo is called a blastocyst.
 |
| Fig. 4.1 |
Zara is now 12 weeks pregnant and has just attended the midwives’ clinic to arrange her 12-week nuchal fold scan at her local maternity unit. Zara is quite excited because not only is her twin sister pregnant but her husband’s sister and two of her best friends as well are pregnant and all the babies are due within the same 2 weeks. They, and their partners, had all been working abroad together doing voluntary work over a period of 1 year and had lived together in shared accommodation.
They had helped build and set up an orphanage for children whose parents had died from AIDS-related illnesses in an African country, and had only returned home a week before Zara’s pregnancy was confirmed.
• What possible explanation could account for all five of the women conceiving together?
• What environmental factors could have influenced the menstrual cycles of the women and what possible physiological mechanisms may have resulted in the group apparently ovulating simultaneously?
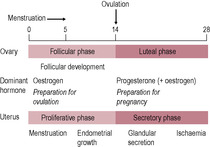
Ovulatory cycles usually have a duration of 24–32 days; the follicular phase is 10–14 days and the luteal phase between 12 and 15 days. Longer cycles usually have a prolonged follicular phase and delayed ovulation.
The follicular phase
Developmental stages
The stages of cell division leading to the production of the female gamete begin early in fetal life. The process is halted until puberty when ovulation begins, and then halted again until fertilization. The developing egg is called an oocyte; it differentiates into a mature haploid ovum (or egg). Although dramatic progress in the development of an oocyte takes place during the follicular phase, the development of the follicle begins about 3 months prior to the menstrual cycle in which it is released at ovulation (Fig. 4.2). Folliculogenesis begins with the recruitment of primordial follicles from the ovarian pool; the signals which initiate recruitment are unknown. At the beginning of the cycle, a number of primary follicles are recruited to undergo initial development, stimulated by luteinizing hormone (LH). These secondary follicles express follicle-stimulating hormone (FSH) receptors. One follicle is selected to continue development as the dominant follicle. It is this follicle that releases the ovum at ovulation. The remnants of the follicle then become the corpus luteum.
 |
| Fig. 4.2 (Reproduced with permission from Brooker, 1998.) |
In the female fetus, the primordial germ cells migrate to the ovary at 21 days postfertilization and become oogonia. They proliferate by mitotic division and then differentiate into primary oocytes; by 20 weeks of gestation, in each ovary, there are about 10 million primary oocytes ready to enter meiotic division (see Chapter 5). The oocytes degenerate throughout fetal life so the female neonate is born with a finite number of 250000–500000 oocytes; this is all the oocytes she will ever have. Degeneration of the oocytes continues throughout postnatal life; there are fewer than 200000 oocytes left at puberty and numbers continue to fall. However, if one assumes a reproductive life of about 35–40 years, the maximum number of ova released from the ovaries will be no more than a few hundred. Although it was thought that the oocytes number was established during fetal development, it is now suggested that some ovarian oogonia still unassociated with follicular cells persist from the fetal period and can undergo mitosis thus acting as a stem cell population of oocytes in postnatal life (Johnson et al., 2004).
Arrested meiosis
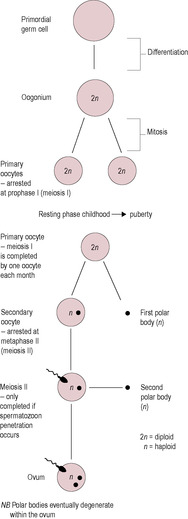
Meiosis is a specialized cell division involved in the production of gametes (see Chapter 7, p. 149, for a detailed description). In it, the number of chromosomes is reduced from 46 (known as the diploid number) to 23 (the haploid number). In the first part of meiosis, the DNA replicates, so each chromosome is composed of two chromatids. The homologous chromosomes pair up along their long axes and crossing over occurs so the chromosomes exchange genetic material. In humans, two or three exchanges of DNA occur per chromosome pair ensuring that the combination of genes will be unique (Box 4.2). This is achieved by areas of fusion (called chiasmata) forming between adjacent chromosomes (see Chapter 7). The oocyte remains arrested in the prophase stage of division I of meiosis for a number of years until sexual maturity (Fig. 4.3). During this time, the primary oocytes synthesize the extracellular matrix and the secretory vesicles. Arrested meiosis provides a specialized mechanism to allow the oocyte to grow while it contains duplicate copies of chromosomes and, therefore, twice as much DNA is available to direct synthesis of RNA as in a somatic cell. The hormones of puberty release the oocyte from the first meiotic block; this process is called oocyte maturation. The oocyte resumes division I of meiosis. The chromosomes re-condense, the nuclear envelope breaks down and the meiotic spindle forms. The chromosomes then segregate into the two daughter nuclei. The cytoplasm divides asymmetrically to form a large secondary oocyte and a small polar body, each of which contains 23 chromosomes as pairs of chromatids. Meiotic division then arrests for a second time, so the oocyte released at ovulation has still not completed meiosis. Activation of the oocyte and release from the second meiotic block occurs at fertilization. In the second meiotic division, the sister chromatids finally segregate into two nuclei and the cytoplasm again divides asymmetrically, this forming the mature ovum and a second polar body, each with 23 chromosomes. Because both divisions of the cytoplasm are asymmetrical, the ovum is large. Effectively, the polar bodies are small packages of cytoplasm which contain discarded chromosomes. During in vitro fertilization (IVF), observing the presence of the second polar body indicates that fertilization has occurred (see Chapter 6).
Box 4.2
• Cell proliferation (mitosis)
• Genetic reshuffling and reduction (meiosis)
• Packaging of chromosomes
• Gamete maturation
 |
| Fig. 4.3 (Reproduced with permission from Brooker, 1998.) |
In the female, mitosis of the gametes stops in the fetal period, whereas in the male, mitotic division of gametogenesis begins at puberty and continues until senescence (see Box 4.3). The termination of mitosis in the female, and entry into meiosis, is under the control of protein complexes, maturation-promoting factor (MPF) and cytostatic factor (CSF), which regulate the oocyte’s progression through meiosis (Dale et al., 1999). MPF phosphorylates multiple proteins involved in chromosome condensation and the breakdown of the nuclear envelope. The hormones of puberty increase MPF activity at the exit of the first meiotic block. It is hypothesized that the second meiotic block occurs because the maturing oocyte accumulates CSF which inhibits cell division. Oocyte activation and the release from the second meiotic block are thought to be triggered by a component of the sperm that enters the oocyte at fertilization and causes CSF degradation.
Box 4.3
• Mitosis: fetal in woman; after puberty in man
• Meiosis: halted in woman; can last many years
• Relatively few oocytes released
• Release is episodic at ovulation; not a continuous stream
• Organization is comparable to testis (stromal tissue containing primordial follicles, tubules) and glandular tissue (interstitial glands, Leydig cells)
• Mitotic proliferation is less
• Time-course of gamete production is much longer
Both premature arrest at the end of oocyte maturation and parthenogenic release from meiotic arrest are thought to cause infertility. As women age, the extended duration of arrested meiosis, which may last for over 50 years, probably results in the meiotic spindles (see Fig. 7.11, p. 153) becoming increasingly fragile; this leads to an increased rate of abnormalities such as Down’s syndrome and failed implantation.
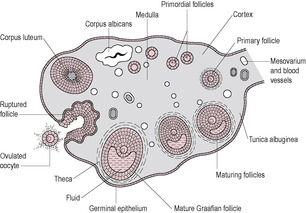
Primordial follicles
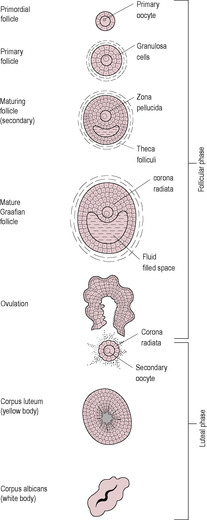
Development of a mature female gamete depends on complex interactions between the developing gamete and the surrounding cells forming the outer layers of the follicle. Mitosis is completed during fetal development. During the first meiotic prophase, the primordial germ cells stimulate organization of the surrounding cells to form the granulosa cells (flattened cuboidal epithelial cells) which condense and encircle them forming the primordial follicles. The follicular cells secrete a basement membrane around the outside forming a cellular unit (Fig. 4.4). These granulosa cells are connected to the oocyte by gap junctions through which nutrients can be transported. The primitive oocyte therefore has two layers and is about 18 μm in diameter. A few follicles may resume development spontaneously and incompletely throughout fetal and neonatal life. However, regular recruitment of the primordial follicles into the pool of growing follicles begins at puberty when levels of FSH increase, so the primordial follicles containing oocytes may stay arrested in meiosis for decades.
 |
| Fig. 4.4 (Reproduced with permission from Brooker, 1998.) |
Preantral (primary) follicles
From puberty, a few primordial follicles spontaneously restart their development each day, forming a continuous stream of growing preantral or primary follicles. Most of these early follicles fail to develop fully and undergo atresia (fail to develop any further); less than 0.1% of follicles will ovulate and develop into a corpus luteum. The granulosa cells of follicles undergoing atresia accumulate lipid droplets and reduce protein synthesis. Both the granulosa cells and the oocyte become apoptotic (undergo ‘programmed cell death’). White blood cells invade the dying follicular cell mass and scar tissue forms.
As the majority of the follicles regress rather than progress through development, the ovary has a dense population of atretic follicles resulting in an irregular corrugated outer surface of the ovary. The development of primordial follicles into primary or preantral follicles takes about 85 days; the preantral phase is the longest phase of development of the oocyte. Initiation and progress through this early follicular development is independent of pituitary hormones but there may be paracrine regulation by cytokines (see Chapter 3), such as epidermal growth factor (EGF; Box 4.4). These developing follicles do not secrete significant amounts of steroid hormones. Further follicular development requires pituitary support (secretion of FSH and LH).
Box 4.4
Inhibin and activin
The gonadotrophins (FSH and LH) stimulate the production of the cytokines, activin and inhibin. The cytokines modulate actions of steroid hormones and gonadotrophins. Inhibin appears to affect only reproduction (see Fig. 4.7), whereas the closely related activin affects cell growth and differentiation in other tissues. Inhibin is produced by the granulosa cells of small antral follicles in response to FSH (Roberts et al., 1993) and suppresses FSH secretion. It is also produced by the Sertoli cells of the testis. Levels peak mid-cycle but remain high in the luteal phase, because the corpus luteum produces inhibin in response to LH. Inhibin production in the pituitary has a local inhibitory effect on FSH release (Hillier, 1991). Inhibin stimulates androgen output by the thecal cells and moderates aromatizing activity of the granulosa cells (Hillier, 1991). Activin is produced by granulosa cells, which also secrete follistatin, which may modulate the effects of activin. The thecal cells of the dominant follicle also produce activin (Roberts et al., 1993). The anterior pituitary gland also produces activin, which is co-secreted with the gonadotrophins and enhances FSH production. It inhibits pituitary production of growth hormone GH, ACTH and PRL. It may also have a role in embryogenesis (Hamilton-Fairley and Johnson, 1998). Activin is present in follicular fluid but is inhibited by follistatin. Activin suppresses the androgen output by thecal cells but stimulates aromatizing capacity of granulosa cells. It therefore inhibits progesterone production. Activin is present early in the cycle, and inhibin later in the cycle, thereby producing a balance between androgen output and conversion.
Follistatin
Follistatin was identified in 1987 as an inhibitor of FSH secretion (Roberts et al., 1993). It is synthesized in the ovary and inhibits activin activity by acting as an activin-binding protein.
Interleukins
Interleukins are a group of cytokines, small protein molecules, that communicate between the immune system cells and between immune and other cells. They are usually secreted by immune cells and usually have a local action. Interleukins include histamine, prostaglandin, tumour necrosis factor (TNF), IL-1 and IL-6. IL-1 is a polypeptide cytokine, usually produced by activated macrophages, which induces an acute phase reaction in the liver in response to inflammation. However, it is also produced by granulosa cells in a hormone-dependent manner with a peak production mid-cycle. IL-1 affects follicular maturation and a number of aspects of ovulation, including increasing production of prostaglandins, collagenase, nitric acid and hyaluronic acid (Hurwitz et al., 1992) and steroidogenesis. It is not known whether other members of the interleukin family are involved in follicular development.
Epidermal growth factor (EGF)
EGF is produced by many tissues including granulosa cells. It appears to inhibit FSH-stimulated oestrogen and inhibin production and proliferation and differentiation of granulosa cells. EGF may be involved in the selection of the dominant follicle (Hamilton-Fairley and Johnson, 1998).
Transforming growth factors (TGF)
Transforming growth factors, TGF-α and TGF-β, have been identified in thecal cells (Adashi et al., 1989). TGF-α has similar properties to EGF and suppresses granulosa cell differentiation. It also regulates differentiation of other cell types including fetal ovaries and ovarian carcinoma cells. Members of the TGF-β family are structurally similar to inhibin and increase FSH receptor expression, positively modulating granulosa cell proliferation and differentiation.
Insulin-like growth factors (IGFs)
Insulin-like growth factors stimulate mitotic division and cell differentiation. Their effects are mediated by insulin-like growth factor binding proteins (IGF-BP). In follicular development, they appear to coordinate the production of steroid hormones from the granulosa and thecal layers of the follicle (Giudice, 1994). IGF-I enhances follicular development and hormone production. IGF-II enhances the response to insulin. IGF-BP bind to the IGFs decreasing the concentration of free growth factor. Decreased levels of binding proteins are associated with follicular growth and increased concentrations of binding proteins are found in atretic follicles (Mason et al., 1993). The large follicles from women with polycystic ovaries have lower concentrations of growth factors and higher concentrations of binding proteins (Mason et al., 1994). IGFs seem therefore to have an important role in follicular growth, maturation and ovulation. In pregnancy, IGFs and their binding proteins play essential roles in modulating fetal growth and development (see Chapter 9).
Tumour necrosis factor (TNF)
TNF was initially identified as having a role in inflammation and in inhibiting tumour growth. It is produced by follicular cells and stimulates steroidogenesis, and may have a role in ovulation.
Early in the cycle, the concentration of FSH is sufficient to support the further development of some preantral follicles. The preantral follicles that are optimal for further development are of the appropriate size and maturity to respond and have adequate FSH receptors. Recruitment of the follicles is related to the interaction between the FSH concentration and the number of FSH receptors on the developing follicles. Therefore, the number of follicles surviving is related to the amount of FSH present. The antral development phase, ovulation and luteal phase comprise one cycle in the humans. In other mammalian species, antral expansion occurs in the luteal phase of the previous cycle, thus shortening non-fertile cycles.
Antral (secondary) follicles
Usually about 15–20 preantral follicles are rescued from atresia each month and undergo initial stages of development and marked enlargement in response to the increasing FSH concentration at the beginning of each cycle. Several components contribute to the growth of the follicle: the oocyte enlarges, the follicular cells divide and further stromal cells are recruited to form the expanded outer layers of the follicle. The oocyte itself increases in diameter to 60–120 μm. It synthesizes large amounts of ribosomal RNA (rRNA) and messenger RNA (mRNA) to increase its protein stores ready for the maturation of the oocyte and fertilization, but does not resume meiosis. The follicular cells divide into several layers of granulosa cells, which secrete an amorphous and acellular translucent jelly, the zona pellucida. The zona pellucida is formed from condensation of glycoprotein and accumulates between the granulosa cells and the oocyte, acting as an extracellular coat of the oocyte. It has an important role in sperm binding and penetration during fertilization (see Chapter 6). Although the zona pellucida acts to separate the oocyte from the avascular granulosa cells, cytoplasmic processes penetrate the zona pellucida forming gap junctions at the oocyte surface. These allow delivery of low molecular weight substrates, such as nucleotides and amino acids, and cellular signalling molecules into the oocyte. Gap junctions also exist between granulosa cells.
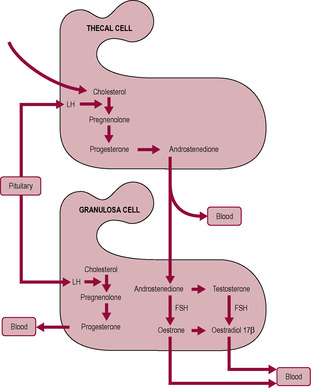
The third component of follicular growth is condensation of ovarian stromal cells on the basement membrane (membrana propria) of the follicle. These recruited cells form a loose matrix of spindle-shaped cells around the follicle, known as the thecal layer. The cells differentiate into two layers: the theca interna, an inner layer of highly vascular glandular cells, and the theca externa, a poorly vascularized fibrous capsule.
There is critical bidirectional paracrine and juxtacrine communication between the oocyte and the surrounding somatic or follicular cells of the ovary, the granulosa and thecal cells. FSH is required both for granulosa cell differentiation and division (Richards and Pangas, 2010). The granulosa cells acquire receptors for FSH and oestrogen and the theca interna cells acquire receptors for LH. Synthesis of steroid hormones by the follicle requires cell cooperation (Fig. 4.5; Hillier et al., 1994). Interstitial glands lie within the stroma and between the developing follicles. They are formed of steroidogenic cells and produce androgens for secretion and aromatization to oestrogen in follicles. LH stimulates the theca interna cells to synthesize androgens (testosterone and androstenedione) from acetate and cholesterol but these cells initially have limited capacity to synthesize oestrogens. Androgens from the theca interna cells diffuse to the avascular granulosa cells. The granulosa cells are unable to synthesize androgens but can aromatize androgens to oestrogens (oestradiol-17β and oestrone). The enzyme aromatase (CYP19) is involved in the steroid biosynthesis pathway leading to increased oestrogen production. FSH stimulates production of insulin-like growth factor I (IGF-I), which stimulates aromatase activity and hence oestrogen production. Activins and oestradiol enhance the actions of FSH (Richards and Pangas, 2010). Small amounts of LH are required to amplify follicular oestrogen production. The steroids are secreted into the bloodstream, where they have a systemic effect, and into the follicular fluid, where they may have a paracrine role. Androgens also stimulate aromatase. Oestrogens stimulate granulosa cells to proliferate and express further oestrogen receptors. Therefore, oestrogen further stimulates oestrogen output, an example of positive feedback. This increases the amount of circulating oestrogen from the most advanced or ‘dominant’ follicle (therefore, monitoring oestrogen output is a guide to the maturity of the most mature follicles). The dominant follicle, which undergoes the most growth, will enlarge from 20 to 200–400 μm diameter. The dominant follicle produces oestradiol, which inhibits FSH secretion. However, the dominant follicle develops exquisite sensitivity to FSH and can continue to respond to the decreasing concentration of FSH. The smaller follicles, destined to become atretic, lose their responsiveness to FSH and do not develop LH receptors (Scheele and Schoemaker, 1996).
Early in the antral phase under the influence of FSH, the granulosa cells produce inhibin B and activin which suppresses androgen output and increases the aromatizing capability of the granulosa cells, thus promoting oestrogen synthesis. Later in the antral phase under the influence of both FSH and LH, the granulosa cells switch to producing inhibin A which stimulates androgen output and attenuates the aromatizing activity. Thus, androgen output is regulated by activin and inhibins and the ratio of inhibin A:inhibin B acts as a marker of follicular growth
The dominant antral follicle
The single follicle emerging as dominant undergoes preovulatory growth. This dominant follicle produces more oestrogen, which inhibits production of FSH from the pituitary gland. This is an example of negative feedback where a product limits its own production. The effect of oestrogen inhibiting production of FSH is that further development of the other follicles is limited. These follicles with fewer FSH receptors exposed to a diminishing supply of FSH are least able to respond to FSH and therefore undergo a downward spiral and become atretic. The dominant follicle continues to produce inhibin A which stimulates androgen production by the thecal cells and aromatization by the granulosa cells, thus leading to the surge of oestrogen. The dominant follicle also has a high ratio of insulin-like growth factor 2 (IGF-2) to IGF-BP which mediates LH-stimulated androgen output and FSH-dependent aromatization.
Angiotensin II (A-II), the product of the renin–angiotensin system (RAS, see Chapter 2), may be involved in oocyte maturation and ovulation. There is a RAS that is specific to the ovary and A-II occurs in high concentrations in preovulatory follicles. This ovarian RAS is implicated in the pathogenesis of ovarian tumours, ovarian hyperstimulation syndrome, ectopic pregnancy and hypertension (Hassan et al., 2000). Symptoms of ovarian hyperstimulation syndrome include serious metabolic and fluid disturbances which are associated with abnormal control of the RAS (note that ovarian hyperstimulation syndrome is a recognized complication of IVF treatment – see Chapter 6). It has also been suggested that A-II may have a role in the formation and maintenance of the corpus luteum, regulation of progesterone production and angiogenesis (Hamilton-Fairley and Johnson, 1998). The biggest or dominant follicle, which is best able to respond to FSH, further develops on the pathway to expansion and ovulation. Oestrogen and FSH stimulate the mid-cycle expression of LH receptors on the outer layers of granulosa cells of the dominant follicle, which means that it will be able to respond to the mid-cycle surge of LH secretion. Entry into the preovulatory phase depends on both the expression of these receptors and a surge of LH from the anterior pituitary gland.
The granulosa cells continue to divide and increase in size. However, most of the increase in follicular size is due to accumulation of follicular fluid formed from mucopolysaccharides, secreted from granulosa cells, and serum transudate. The fluid coalesces forming an antrum (or cleft) filled with follicular fluid. The antrum separates the granulosa cells into two regions: the corona radiata (a rim of granulosa cells) around the oocyte and the outer membrana granulosa. The oocyte becomes isolated and suspended in the fluid connected to the rest of the granulosa cells by a thin strand of cells, the cumulus oophorus (egg stalk). The oocyte does not increase in size but continues to synthesize RNA and protein.
Follicular development is dependent on pituitary support. Removal of the pituitary gland (hypophysectomy) results in the cessation of follicular growth and the death of the oocyte. This can be halted by adding back LH and FSH, which stimulate further growth. It takes 8–12 days for the primary follicle to grow into the antral follicle. Failure of follicular growth for any reason results in a restarting of the cycle of follicular development, and hence both a longer first phase of the cycle and a longer cycle. It seems likely that women who regularly have a longer than normal menstrual cycle have either a slow rate of follicular development and increasing oestrogen secretion or the dominant follicle starts developing but fails, so the next most appropriate follicle takes over the role as the dominant follicle.
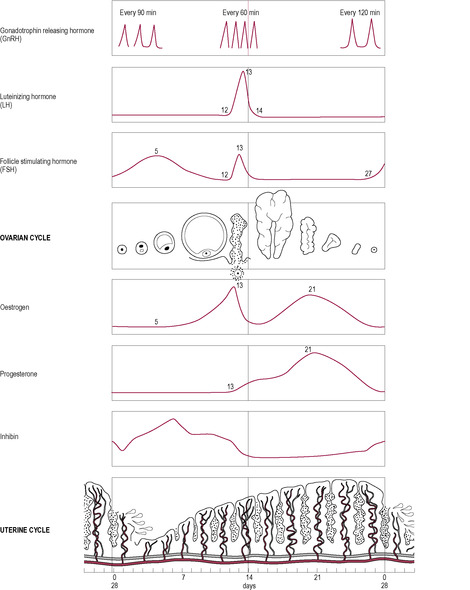
Although the increasing concentration of oestrogen (predominantly oestradiol, E2) initially has a negative feedback on the hypothalamus and pituitary, there is a critical concentration of oestrogen that is stimulatory provided it lasts for a critical duration. When the diameter of the follicle is 18–22 μm and the oestradiol concentration reaches 600–1200 pmol/L, there is positive feedback on the anterior pituitary gland leading to a sudden increase or ‘surge’ of LH release (Hamilton-Fairley and Johnson, 1998; Fig. 4.6).
The LH surge
The effect of the LH surge is twofold. First, it stimulates the terminal growth phase of the preovulatory follicle and the meiotic and cytoplasmic maturation of the oocyte, culminating in expulsion of the oocyte from the ovary. These effects include re-initiation of oocyte meiosis and expansion of the cumulus cell–oocyte complex (Richards and Pangas, 2010). Second, it causes luteinization, a series of endocrine changes within the follicular cells that result in a different hormone secretory profile in the second half of the cycle.
Within a few hours of the LH surge, there are dramatic changes in the oocyte, which resumes meiotic division. There may also be a positive signal from the granulosa cells or a reduction of gap junction communication, which decreases the flow of meiosis-arresting substances to the oocyte. Progression through the remainder of the first meiotic division results in half the chromosomes (as paired chromatids) and almost all the cytoplasm being enclosed in the secondary oocyte, which is destined to become the ovum. The remaining chromosomes and very little cytoplasm are enclosed in a membrane forming a very small cell, known as the first polar body (see Fig. 4.3). Thus, the secondary oocyte keeps the bulk of the materials that were synthesized earlier in follicular development; these are conserved for the zygote. The chromosomes of the secondary oocyte enter the second meiotic division and go on to the next stage of division, called metaphase (see Fig. 7.11), where they align on the spindle. However, meiosis is then immediately arrested for a second time; this is regulated by CSFs. Meiosis resulting in the production of a mature female pronucleus will not resume until successful fertilization following ovulation. By this time, the oocyte will already contain the sperm nucleus. Thus, there is actually no time when the oocyte is a true gamete in the sense of being a cell with only 23 chromosomes, as is the case of a spermatozoon.
Concurrently, the LH surge promotes maturation of the cytoplasmic compartment of the oocyte. The cytoplasmic processes between the oocyte and the granulosa cells withdraw and contact is lost. The Golgi apparatus (see Table 1.1) synthesizes lysosome-like cortical granules, which align under the surface of the oocyte. Protein synthesis continues but the profile of the proteins synthesized changes as the oocyte prepares for fertilization. The gonadotrophin surge stimulates the cumulus cells surrounding the oocyte (see Fig. 4.4) to secrete hyaluronic acid, which disperses the cumulus cells embedding them in a mucus-like matrix.
Ovulation
Ovulation is triggered by the mid-cycle surge of LH, which occurs in response to sustained high levels of oestrogen released from the developing dominant follicle. The single mature preovulatory follicle has a diameter of 2–2.5 cm in an ovary that is approximately 3 cm long. It was this structure that de Graaf identified and named in 1672. The increased size and changed position of the follicle mean that it protrudes from the surface of the ovary (see Fig. 4.2). This results in the thinning of the layer of epithelial cells between the wall of the follicle and the peritoneal cavity. As expansion continues, the wall becomes thinner and avascular, and the cells appear to dissociate.
About 36 h after the LH surge, ovulation occurs and the oocyte is expelled from the ovary (see Fig. 4.2). The LH surge stimulates the production of a cascade of proteolytic enzymes, including renin and other trypsin-like enzymes from thecal cells, which digest the follicle wall. The biochemical changes, including generation of oxygen free radicals, that precede ovulation are similar to those seen in inflammation. Plasminogen activator, which converts procollagenase to collagenase, is produced by granulosa cells resulting in the breakdown of the connective tissue. Progesterone production rises immediately after the LH surge and the preovulatory increase in progesterone may be important in follicular rupture as it decreases formation of collagen. Prostaglandins increase vascular permeability, which maintains the intrafollicular pressure as fluid begins to leak through the eroded follicular wall. Small contractile waves also ripple across the ovary increasing the intrafollicular pressure. The force is cushioned by the follicular fluid, so the pressure generated is targeted at the weakened ovarian surface, causing it to rupture. Some women experience abdominal pain around the time of ovulation on the same side of the ovary producing the ovum. This is referred to as Mittelschmerz pain (derived from German ‘middle pain’). As the follicle ruptures and the ovarian surface is breached, the fluid washes out the oocyte, which is surrounded by the granulosa (cumulus) cells, from the ovary to the exterior. The oocyte is swept into the uterine tube by the fimbria. It is then propelled towards the uterus by peristaltic muscular activity and cilia movements of the epithelial cells lining the tube. After taking years (12–50 years) to complete maturation, the oocyte is then viable and fertilizable for only about a day.
The luteal phase
Within 2 h of the LH surge, there is a transient rise in the oestrogen and androgens secreted by the follicle as the thecal layers become stimulated and hyperaemic. The outer granulosa cells with their newly expressed receptors for LH no longer convert androgens to oestrogen but synthesize progesterone instead. The cells no longer bind oestrogen or FSH. The result is a marked increase in progesterone secretion, which begins several hours before ovulation.
The corpus luteum
After ovulation, the residual parts of the follicle remaining in the ovary collapse into the space and form the corpus luteum (‘yellow body’; see Fig. 4.4). There is some bleeding and fibrotic activity in the cavity, which allows formation of a fibrin core around which the remaining granulosa cells congregate. The structure is enclosed by a capsule of fibrous thecal cells. The basement membrane between the granulosa cells and thecal cells breaks down allowing vascularization of the interior. This allows increased transport of cholesterol precursor to the luteinizing granulosa cells to maintain a high rate of progesterone secretion. A few of the thecal cells disperse to the stroma tissue. The granulosa cells first luteinize, then stop dividing and hypertrophy into large luteal cells. The luteal cells are rich in mitochondria, endoplasmic reticulum and Golgi bodies and have numerous lipid droplets and lutein, a yellow carotenoid pigment.
Hormonal changes
Luteinization is associated with a progressive increment in progesterone secretion from the corpus luteum. The outer thecal cells form a stem cell population of smaller luteal cells which have numerous LH receptors and produce progesterone and androgens. Levels of progesterone rise until the middle of the luteal phase (see Fig. 4.6). The corpus luteum produces oestrogen and inhibin as well as progesterone. All three hormones inhibit secretion of FSH from the anterior pituitary gland and therefore prevent further development of follicles.
It has been suggested that a cause of fertility problems may be inadequate production of progesterone at the time of ovulation and during the subsequent luteal phase. However, exogenous administration of progesterone or human gonadotrophin (hCG) has had limited success in clinical practice. It appears that most women have a proportion of their cycles with a low progesterone output without their overall fertility being affected (Hamilton-Fairley and Johnson, 1998). A shortened luteal phase can lead to intermenstrual bleeding, premenstrual ‘spotting’ and short cycles.
The corpus luteum also synthesizes relaxin, secretion of which peaks in the middle of the luteal cycle (Johnson et al., 1993), probably regulated by LH. Relaxin may be involved in promoting the growth of the myometrium and cervix and growth and secretory activity of the endometrium (Huang et al., 1991).
Effectively, the corpus luteum is an endocrine gland producing oestrogen and progesterone. The LH surge stimulates its growth and activity. Unless fertilization occurs, the life of the corpus luteum is very short, and it undergoes spontaneous luteolysis (degeneration and regression) after about 6 days. The corpus luteum appears to have an age-related decrease in responsiveness to LH (Zeleznik and Hillier, 1996) and so requires progressively more LH for survival. Following the LH surge, LH concentration in the luteal phase is low, so luteolysis will occur. Blood flow to the corpus luteum falls and the follicular tissue becomes ischaemic. The concentrations of oestrogen and progesterone begin to fall as the degenerating corpus luteum stops hormone production. Thus, luteolysis terminates a non-fertile cycle. As the level of oestrogen falls, the inhibition on the hypothalamus will be abrogated and FSH secretion will resume, ready for the next cycle. The atrophying corpus luteum loses its yellow pigment, so it becomes known as a corpus albicans (‘white body’). It gradually contracts over a period of months, leaving a white scar tissue which is absorbed into the stromal tissue of the ovary.
Changes on fertilization
If fertilization occurs, then hCG, which has structural similarities to LH, rescues the corpus luteum from luteolysis, stimulating its further growth and production of steroid hormone up to the 10th week when placental endocrine function becomes established. Human chorionic gonadotrophin has a longer half-life than LH, so it provides a sustained and more intense stimulus. If fertilization occurs, then the concentration of relaxin also continues to rise until the end of the first trimester.
Regulation of gonadotrophin secretion
The brain controls and regulates the ovarian cycle. The gonadotrophs in the anterior pituitary secrete the glycoprotein hormones LH and FSH (which together are known as gonadotrophins). There appear to be two distinct populations of cells in the anterior pituitary, each producing one particular type of hormone; however, fluorescent labelling shows that some cells contain both hormones. Synthesis and secretion of LH and FSH are dependent on gonadotrophin-releasing hormone (GnRH) from the hypothalamus, which acts as the common mediator of influences via the CNS. (GnRH is also known as luteinizing hormone releasing hormone, or LHRH.) The hypothalamus releases GnRH into the hypophysial portal circulation that runs to the pituitary gland.
This pathway means that control of the reproduction can be modulated and affected by other inputs from the higher brain centres. The GnRH neurons convert neural signals into endocrine signals. Stress, nutritional status and environmental influences, for instance, affect the timing and success of the reproduction. Ovulation appears to be seasonally regulated in populations experiencing seasonal variation in food availability; suspending reproductive function when nutrition is poor favours maternal health and outcome of pregnancy.
There are two levels of regulation. First, the GnRH neurons of the hypothalamus have an inherent pulsatile activity. The steroid hormones, the gonadotrophins (LH and FSH) and GnRH feedback on the hypothalamic–pituitary axis exert a second level of endocrine control. Prolactin (PRL) also has an effect on the control of reproduction.
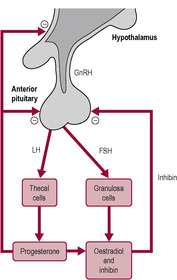
GnRH and gonadotrophins are released in a pulsatile manner (see Box 4.5). The cells releasing GnRH appear to be widely and diffusely distributed (Rance et al., 1991) but are remarkably synchronized to produce pulses of GnRH. During the follicular phase, the pulses are of low amplitude and high frequency, occurring every 60 min (Clarke, 1996). In the luteal phase, they are more irregular and have high amplitude and occur with a low frequency of about every 2 h. The output of the gonadotrophins, LH and FSH, is changed by increasing or decreasing the amplitude or frequency of the pulses or by modulating the response of the gonadotrophs to the pulses. Prior to the LH surge, gonadotroph GnRH receptor density increases and the cells become more sensitive to GnRH. Inhibin and activin affect secretion of FSH without affecting secretion of GnRH. Two phenomena are observed: first, a depressant effect on output of gonadotrophins by increased oestrogen, progesterone and inhibin; and second, an increased surge of LH and FSH secretion induced primarily by oestradiol (Fig. 4.7). The pattern of pulsatile secretion of GnRH is regulated by a complex mechanism that allows multiple signals, such as neurotransmitters and sex steroids, to determine ovulation.
Box 4.5
The study of biological rhythms is termed ‘chronobiology’. All living cells, organs, organisms and groups of individuals demonstrate rhythmical changes within their internal (endogenous) physiology that can also result in external changes in behaviour. The rhythms can be categorized according to the length of the cycle or period of oscillation.
• Ultradian: the rhythm is less than 1 day, for example, rapid eye movement in sleep.
• Circahordal: the period of oscillation is around 1 h.
• Circadian: the period of oscillation is about 1 day; levels of many hormones such as cortisol fluctuate on a daily basis.
• Infradian: the rhythm is repeated in a cycle greater than 1 day, for example, menstrual and oestrus cycles.
• Circaseptram: the period of oscillation is about 1 week.
• Circatidal: the rhythm relates to tidal movement of water.
• Circalunar (synodic): the rhythm relates to the cycle of the moon.
• Circannual: the rhythm has a cycle of about 1 year.
These fluctuations are often affected by the external environment and appear to enable the individual to respond to forthcoming changes within the environment. Factors that influence or reset the cycle are described as entraining the cycle. In the human brain, the suprachiasmatic nuclei of the hypothalamus may influence daily fluctuations. The pineal gland, which produces the hormone melatonin at night, appears to affect the suprachiasmatic nuclei, acting as an entrainer. The circadian pattern of melatonin secretion is achieved through inhibition via a neural pathway (outside the optic nerve) that is activated by light stimulation upon the retina. Therefore, within temperate zones, light acts as an entrainer on a daily basis and, because of the fluctuation of the photoperiod (length of daylight exposure), entrainment of annual cycles can also be achieved.
 |
| Fig. 4.7 (Reproduced with permission from Johnson and Everitt, 1995.) |
In the early part of the follicular phase, rising levels of FSH stimulate oestrogen production from the developing follicles. Rising concentrations of oestradiol have a negative feedback effect on gonadotrophin production from the anterior pituitary, so FSH secretion falls. Activin and inhibin also affect FSH secretion. Inhibin is involved in the negative feedback of FSH secretion. However, the dominant follicle is exquisitely sensitive to even a diminishing concentration of FSH and continues to produce oestrogen, which markedly increases by two- to fourfold. These concentrations are maintained for 48 h, which produces a positive feedback effect resulting in the dramatic surge of LH and FSH release seen in mid-cycle prior to ovulation. The effect of oestradiol is very sensitive: a low concentration has a marked and rapid effect that is evident within 1 h and maximal within 4–6 h. During the luteal phase, increased progesterone concentrations reinforce the negative feedback effects of oestradiol. The production of both LH and FSH secretion is very low; therefore, the positive effect of oestradiol is blocked.
Cyclical effects of oestrogens and progesterone
Effects on the uterus
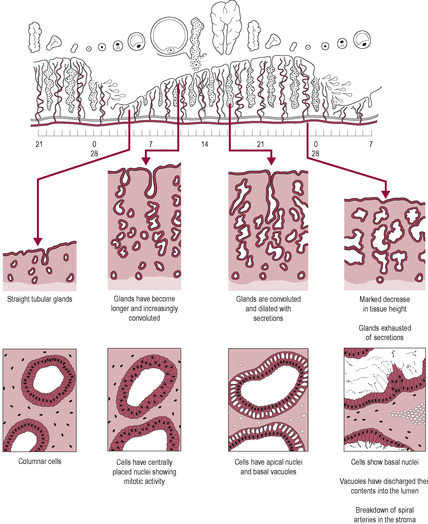
Organs that respond to hormonal changes have receptors for the hormones. Responses can change because hormone levels fluctuate or because receptor density on the target organs alters. The principal actions of oestrogen and progesterone during the monthly cycle are on the endometrium which is one of the tissues most sensitive to ovarian steroid hormones. The endometrium undergoes cyclical changes: the growth of the uterine wall in expectation of an embryo, and its degeneration if fertilization does not take place. In the first half of the cycle, the uterus goes through a proliferative phase. Oestrogen stimulates the epithelial cells of the basal layer of the endometrium to divide and proliferate, forming a thick mucosal wall with numerous endometrial glands (Fig. 4.8). Oestrogen also stimulates proliferation of the stoma and glands and angiogenesis (growth of new blood vessels): extensive vascular tissue, spiral arteries and veins develop within the endometrium. Within the space of a few days, the effect of oestrogen is to increase the height of the wall from 0.5 to 5 mm, a remarkable 10-fold increase. The thickness of the endometrium is monitored by ultrasound in assisted conception (see Chapter 6) to assess whether it is optimal for implantation; the insertion of embryos into a uterine cavity with an endometrium less than 5 mm thick is unlikely to be successful. The myometrium does not grow extensively during the menstrual cycle. During the proliferative phase, oestrogen primes the endometrial cells by inducing the synthesis of progesterone receptors.
 Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|