4 Recovery and Rehabilitation
After reading this chapter, you should be able to:
• discuss the physical, psychological and cognitive sequelae present for some survivors of a critical illness
• outline the common functional, psychological and health-related quality of life (HRQOL) instruments used to assess patient outcomes after a critical illness
• describe the benefits and challenges for implementing rehabilitation interventions in-ICU, in hospital after ICU-discharge, and after hospital discharge
Introduction
A critical illness requiring admission to a general intensive care unit (ICU) affects approximately 113,000 adults in Australia and 17,000 in New Zealand per year.1 Although survival rates approximate 89% at hospital discharge,2 functional recovery for individuals is delayed often beyond six months post-discharge.3–5 Physical de-conditioning and neuromuscular dysfunction6,7 as well as psychological sequelae8 are common, adding to the burden of illness for survivors, carers, the health care system and broader society.9
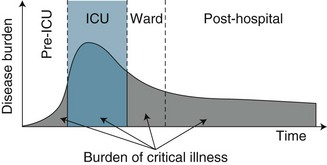
While ICU clinicians have traditionally focused on survival as the principal indicator of patient outcome and unit performance,9 physical and psychological functioning and health-related quality of life (HRQOL) have now emerged as legitimate patient outcomes from both practice and research perspectives.4 With this shifting focus towards long-term health and wellbeing has also come a reconsideration and re-conceptualisation of critical care as only one component in the continuum of care for a critically ill patient. An episode of critical illness is now viewed as a continuum that begins with the onset of acute clinical deterioration, includes the ICU admission, and continues until the patient’s risk of late sequelae has returned to the baseline risk of a similar individual who has not incurred a critical illness9 (see Figure 4.1). Timing of this recovery trajectory is variable, and related to a number of individual, illness and treatment factors.
Reviews of numerous observational studies confirm delayed recovery in HRQOL,e.g.3–5 with both physical and psychological symptoms prevalent:
The effects of a critical illness on cognitive functioning are now also beginning to be examined and discussed in the literature as an important patient outcome.11,15–19 While significant sequelae therefore exist for a substantial proportion of critical illness survivors, little evidence is currently available to support specific interventions for improving their recovery.9,20
A further and more recent re-conceptualisation of holistic critical care practice promotes a unifying approach for minimising intensive care unit acquired weakness (ICU-AW) and delirium, reflected in the acronym ABCDE,11,21 to minimise physical, psychological and cognitive sequelae:
B Breathing trials (to minimise mechanical ventilation duration)
C Coordination (of daily awakening and spontaneous breathing trials)22
E Exercise/Early mobility (requires a patient to be awake, alert and co-operative).
Further chapters in this book discuss psychological issues including sedation management and delirium monitoring while in ICU (Chapter 7), and breathing trials and weaning from mechanical ventilation (Chapter 15). This chapter discusses common physical and psychological sequelae associated with a critical illness, and how this impacts on a survivor’s HRQOL. Common instruments measuring physical, psychological and HRQOL are described. Physical rehabilitation strategies, commencing with exercise and early mobility in-ICU, post-ICU and post-hospital services are also discussed.
Icu-Acquired Weakness
Critical illness myopathy (CIM), polyneuropathy (CIP) and neuromyopathy (CINM) syndromes23 occur in 46% of ICU survivors.6 More recently, ICU-Acquired Weakness (ICU-AW) has been proposed as a term to encompass these syndromes of muscle wasting and functional weakness in patients with a critical illness who have no other plausible aetiology.24 The three syndromes above form the sub-categories of ICU-AW, with CINM used when both myopathy and axonal polyneuropathy are evident. Development of ICU-AW is associated with a number of risk factors:24–26
• co-existing conditions: chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus
• critical illness: sepsis, systemic inflammatory response syndrome (SIRS)
• treatments: mechanical ventilation, hyperglycaemia, glucocorticoids, sedatives, neuromuscular blocking agents, immobility.
Local and systemic inflammation acts synergistically with bed rest and immobility to alter metabolic and structural function of muscles,27 resulting in muscle atrophy and contractile dysfunction,26 loss of flexibility, CIP, heterotopic ossification and entrapment neuropathy.6 Muscle strength can reduce by 1–1.5% per day with a total loss of 25–50% of body strength possible following immobilisation.28 Patients can lose 2% of muscle mass per day, which contributes to weakness and disability, and a prolonged recovery period.25 These neuromuscular dysfunctions are diagnosed by clinical assessment, diagnostic studies (electrophysiology, ultrasound) or histology of muscle or nerve tissue.24
The syndrome manifests as prolonged weaning time, inability to mobilise and reduced functional capacity. Some groups of ICU survivors report relatively poor HRQOL due to prolonged weakness that may persist for months and years after discharge, particularly for those recovering from Acute Lung Injury/Adult Respiratory Distress Syndrome.29–31 A related factor is nutrition, with one study noting that 39% of patients post-ICU had little or no appetite and 15% were still receiving either a soft diet or tube feeding while in hospital.32
Clinical Assessment
Clinical assessment includes identification of generalised weakness following the onset of a critical illness, exclusion of other diagnoses (e.g. Guillain–Barré syndrome), and measurement of muscle strength. Patients suspected of ICU-AW have diffuse flaccid weakness that is symmetrical and involves both proximal and distal muscles, with relative sparing of cranial nerves and variable deep tendon reflex responses.23
Manual Muscle Testing (MMT) is commonly assessed using the Medical Research Council (MRC) Scale,33 a 0–5 point ordinal scale:
1 = flicker or trace of muscle contraction
2 = active movement with gravity eliminated
3 = reduced power but active movement against gravity
4 = reduced power but active movement against gravity and resistance
• upper limb: deltoid, biceps, wrist extensors
• lower limb: quadriceps, gluteus maximus, ankle dorsiflexion34
Weakness is evident with an MRC total score of <48 (<4 in all testable muscle groups), and re-tested after 24 hours. Weakness (<4 MRC Scale) was associated with an increased hospital mortality.34 Inter-rater reliability following appropriate training using the MRC has been demonstrated.35
Hand-held dynamometry enables measurement of grip strength force using a calibrated device for patients who are conscious and cooperative. Dynamometry was demonstrated to be a reliable, rapid and simple alternative to comprehensive MMT assessment,34 and may be a surrogate measure for global strength.24
Diagnostic Testing
Electrophysiological testing (nerve conduction studies, needle electromyography) may be useful as an adjunct in diagnosing ICU-AW, but differentiating between CIM and CIP is difficult.24 Muscle wasting is a consequence of inflammatory responses (including COPD-associated inflammation).25 Histology for CIP is primarily noted as distal axonal degeneration in both sensory and motor fibres, while the characteristic findings in CIM is patchy loss of myosin (thick filaments), necrosis and fast twitch fibre atrophy.24 Ultrasound is also being examined as a reliable assessment of muscle mass/volume in this cohort, although findings can be confounded by tissue oedema.24
Practice tip
Current clinical recommendations to limit muscle wasting include:
• minimising patient exposure to corticosteroids and neuromuscular blocking agents
• limiting excessive analgesia and sedation
• glycaemic control may also be of value, although further investigations continue
• early nutrition or specific nutritional supplements or components may limit loss of muscle mass or enhance muscle recovery, but also requires further research.25
Patient Outcomes Following a Critical Illness
Examination of patient outcomes beyond survival is an important contemporary topic for critical care practice and research.3–536 Patient outcomes after a critical illness or injury were traditionally measured using a number of objective parameters (e.g. number of organ failure-free days, 28-day status, or 1-year mortality).37 Other measures that examined patient-centred concepts such as functional status and HRQOL38,39 have become more prevalent in the literature.3,4,40–42 As the recovery trajectory from a critical illness may be long and incomplete, mapping this path is a complex process. The range of HRQOL instruments available is large, but can be divided into two groups: generic to all illnesses, or specific to a particular disease state. One limitation of generic instruments is that, while they can be applied to a broad spectrum of populations, they may not be responsive to specific disease characteristics.43 This section discusses the measurement of health outcomes, focusing on HRQOL, and the physical and psychological measures commonly used to assess survivors of a critical illness.
As introduced earlier, reviews of numerous observational studies with survivors of a critical illness have demonstrated a delayed recovery trajectory, highlighting particularly the effect of physical function on an individual’s usual role. Recommendations for future studies noted that patients should be followed for at least six months, have neuropsychological testing as part of their assessment, and be assessed using a HRQOL instrument that enables comparison across countries and languages.3,9,44,45 Common instruments used to assess HRQOL, physical functioning and psychological functioning for cohorts of patients after a critical illness are discussed below.
Measures of Health-Related Quality of Life after a Critical Illness
A generic instrument that measures baseline HRQOL and exhibits responsiveness in a recovering critically ill patient with demonstrated reliability and validity has been elusive, although recent review papers have identified some useful instruments4,9 (see Table 4.1). SF-36 is the most commonly used and validated instrument in the literature, including with a variety of critically ill patient groups (e.g. general ICU, ARDS, trauma and septic shock). A recent comparison of two related instruments demonstrated that the 15D was more sensitive to clinically important differences in health status than EQ-5D in a critical care cohort.46
TABLE 4.1 Summary of health-related quality of life (HRQOL) instruments used for patients following a critical illness
| Instrument | Items | Concepts/domains |
|---|---|---|
| Medical outcomes study (SF-36)162,163 | 36 | physical: functioning, role limitations, pain, general health; mental: vitality, social, role limitations, mental health; health transition; variable response levels (2–5) |
| EuroQol 5D46,164 | 5 | mobility, self-care, usual activities, pain/discomfort, anxiety/depression; 3 response levels; cost-utility index |
| 15D46,165 | 15 | mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort, distress, depression, vitality, and sexual activity; 5-point ordinal scale (1 = full function; 5 = minimal/no function) |
| Quality of life–Italian (QOL–IT)166 | 5 | physical activity; social life; perceived quality of life; oral communication; functional limitation; varied response levels (4–7) |
| Assessment of Quality of Life (AQOL)167 | 15 | Illness (3 items); independent living (3 items); physical senses (3 items); social relationships (3 items); psychological wellbeing (3 items); 4 response levels; enables cost-utility analysis |
| Quality of life–Spanish (QOL–SP)168 | 15 | basic physiological activities (4 items); normal daily activities (8 items); emotional state (3 items) |
| Sickness impact profile (SIP)169 | 68 | physical: somatic autonomy; mobility control; mobility range psychosocial: psychic autonomy and communication; social behaviour; emotional stability; developed from original 136-item170 |
| Nottingham Health Profile (NHP)171 | 45 | experience: energy, pain, emotional reactions, sleep, social isolation, physical mobility; daily life: employment, household work, relationships, home life, sex, hobbies, holidays |
| Perceived quality of life (PQOL)172 | 11 | satisfaction with: bodily health; ability to think/remember; happiness; contact with family and friends; contribution to the community; activities outside work; whether income meets needs; respect from others; meaning and purpose of life; working/not working/retirement; each scored on 0–100 scale |
Measures of Physical Function Following a Critical Illness
A variety of instruments have been developed to examine the physical capacity of individuals, usually focusing on functional status ranging from independent to dependent. Table 4.2 describes some common instruments used with individuals after an acute or critical illness. Many other instruments exist for specific clinical cohorts, including Katz’s ADL index,47 the Karnofsky performance status,48 and the instrumental activities of daily living,49 but these have not been used commonly with survivors of a critical illness.
TABLE 4.2 Common measures of physical function following a critical illness
| Instrument | Measurement | Score range/comments |
|---|---|---|
| St George’s Respiratory Questionnaire (SGRQ),173 (SGRQ-C)174 | COPD-specific items assessing three domains: symptoms (7 items), activity (2 multi-part items), impacts (5 multi-part items) | Item responses have empirical weights; higher scores indicate poorer health; used with patients with chronic lung disease, including ARDS |
| Six-minute walk test (6MWT)51 | Walk distance, reflects functional capacity in respiratory or cardiac diseases | Assesses walk function in patients with moderate heart failure, ARDS |
| Barthel Index (BI)175–177 | 10 items of functional status (Activities of Daily Living [ADLs]) | Dependence: total = 0–4; severe = 5–12; moderate = 13–18; slight = 19; independent = 20 |
| Functional Independence Measure (FIM)178 | Severity of disability in inpatient rehabilitation settings | 18 activities of daily living in two themes: motor (13 items), cognitive (5 items); 7-point ordinal scales; score range 18–126 (fully dependent–functional independence) |
| Timed Up and Go (TUG)179 | Functional ability to stand from sitting in a chair, walk 3 m at regular pace and return to sit in the chair | ≤10 seconds = normal; ≤20 seconds = good mobility, independent, can go out alone; 21–30 seconds = requires supervision/walk aid |
| Shuttle walk test (SWT)180 | 10 m shuttle walk with pre-recorded audio prompts to complete a shuttle turn | Participant keeps pace with audio sounds; 12 levels of speed (0.5–2.37 m/second) |
ARDS = Adult Respiratory Distress Syndrome
Physical activity associated with cardiac or pulmonary dysfunction may be assessed using perceived breathlessness (dyspnoea) during exercise by the modified Borg scale,50 ranging from 0 (no dyspnoea) to 10+ (maximal). The Borg scale is commonly used with other physical activity instruments, e.g. the six-minute walk test (6MWT).51
Measures of Psychological Function after a Critical Illness
The recovery process and trajectory for survivors of a critical illness remains an important but under-researched area.18,52 Exploration of the impact of the intensive care experience, including ongoing stress53–56 and memories for the patient,16,57–59 is now emerging in the literature as an important area of research and practice. Instruments that assess mental function after a critical illness focus on psychological constructs, including anxiety, avoidance, depression and fear (see Table 4.3). Other instruments are also available to examine post-traumatic stress symptoms.60 Constructs that relate to an individual during a critical illness episode also include agitation, and confusion/delirium14 (discussed further in Chapter 7).
TABLE 4.3 Examples of common measures of psychological function after critical illness
| Instrument | Measurement | Score range |
|---|---|---|
| Impact of event scale (IES);181 IES-R182 | 15-item; assesses levels of post-traumatic distress; two subscales: intrusive thoughts, avoidance behaviours; revised form (IES-R) adds hyper-arousal subscale (7 items)182 | frequency of thoughts over past 7 days; 0 = no thoughts; 5 = often; higher scores indicate greater distress: scores ≥26 (combined intrusion and avoidance) are significant |
| Hospital anxiety and depression scale (HADS)89 | 14 items; 4-point scale; measures mood disorders in non-psychiatric patients; focuses on psychological rather than physical symptoms of anxiety and depression | combined score ≥11 indicates a clinical disorder |
| Center for Epidemiologic Studies–Depression Scale (CES–D)183 | 20-item self-report scale assessing frequency and severity of depressive symptoms experienced in the previous week | score range 0–60; higher scores reflect increased symptoms and severity |
Assessment for ongoing neuro-cognitive dysfunctions17,61,62 is recommended for some survivors, with the beginning of research on cognitive rehabilitation for survivors of a critical illness evident.15,17–19,63 Cognitive executive functioning includes attention, planning, problem-solving and multi-tasking.64
Psychological Recovery
Psychological responses to a critical illness and patients’ memories of experiences during an ICU admission have been explored using quantitative57,65–69 and/or qualitative approaches.70,71 Some survivors reported increased anxiety, including transfer anxiety (discharge from ICU);32 depression;13 post-traumatic stress;14,72–74 hallucinations;58,75,76 and continuing cognitive dysfunction.77 A range of memories and experiences were also noted after ICU transfer78and hospital discharge,58,70,79 including powerlessness, reality–unreality, reactions and acceptance, and comfort–discomfort.
For some patients, recovery from a critical illness results in short- and long-term psychological dysfunction (e.g. anxiety, depression and posttraumatic stress symptoms).8,80 Our understanding of these sequelae has improved over the last decade in part due to increased research activity and evaluations of intensive care follow-up clinics in the UK (discussed in a later section). Importantly, negative psychological consequences of intensive care can result in poorer health status and perceptions of HRQOL.81
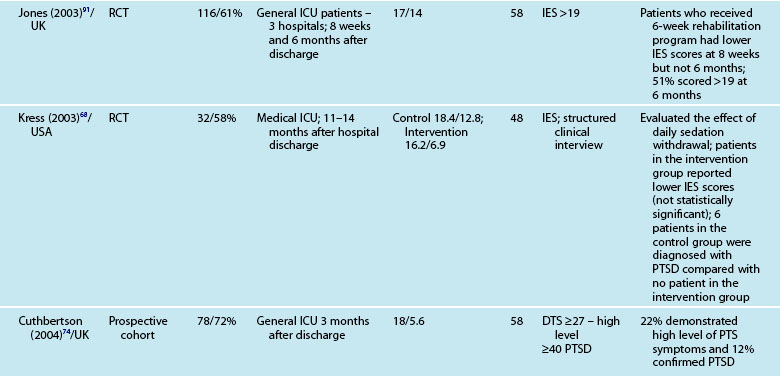
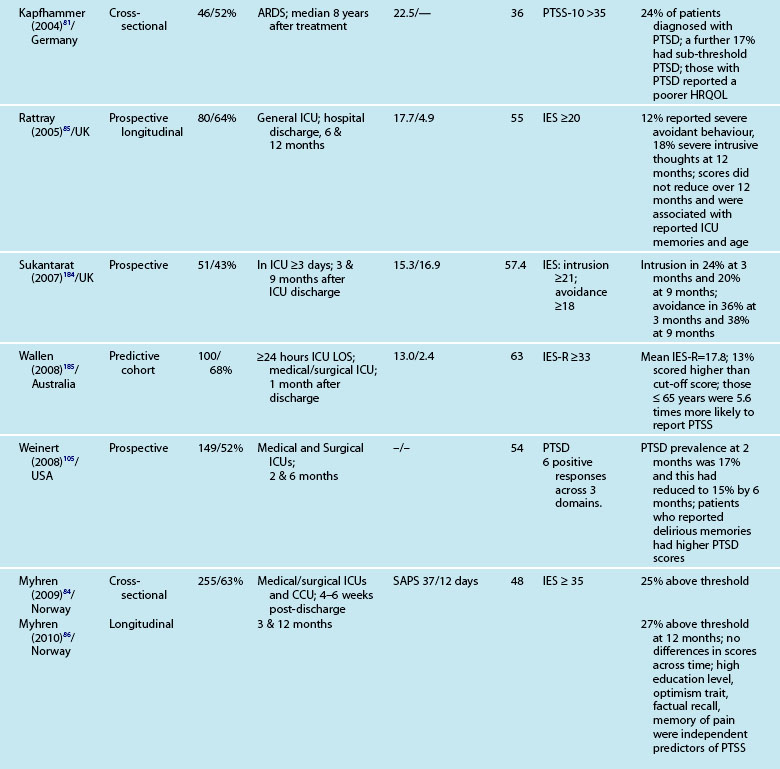
Assessment of psychological outcomes has mainly relied on self-report questionnaires administered via either a postal survey or a structured interview format. These screening, rather than diagnostic, strategies enable identification of individuals at risk of developing a significant clinical problem. A number of standardised questionnaires have demonstrated reliability and validity in this patient group, but the use of different questionnaires makes it difficult to generalise findings. Studies that assessed anxiety and depression used the Hospital Anxiety and Depression Scale (HADS),57,82–86 Beck Anxiety Inventory,87 State Trait Anxiety Inventory (STAI),68 and the Beck Depression Inventory.68,87 Posttraumatic stress has been assessed using the Impact of Event Scale (IES),57,68,84,85 Post-Traumatic Stress Syndrome 10-Questions Inventory (PTSS-10),73 Davidson Trauma Scale,74 and the Experience after Treatment in Intensive Care 7 (ETIC-7) item scale.88
These instruments often include ‘cut-off’ or ‘threshold’ scores that enable screening for the presence or severity of a disorder. For example, a score of 8–10 on either subscale of the HADS indicates possible presence of a disorder, while a score of 11 or above indicates probable presence of such a condition.89 One limitation of these self-report measures is that while sensitivity (ability to correctly identify all patients with the condition) can be high, specificity (ability to correctly identify all patients without the condition) is less easy to determine, and therefore the incidence of psychological distress may be over-stated. This makes estimation difficult and is one of the challenges in establishing the actual magnitude of psychological distress after a critical illness. Other challenges include the recruitment of different cohorts or subgroups of patients (e.g. patients with Adult Respiratory Distress Syndrome87 or Acute Lung Injury90). Variations in the international provision of ICU services also means that differences may exist in case mix in the areas of illness severity, planned or unplanned admissions, ages and reasons for admission. For example, in a sample of studies mean age ranged from 4090–59 years,91 mean APACHE II scores ranged from 1583–24.9,92 and median length of ICU stay from 3.783–3487 days.
Anxiety and Depression
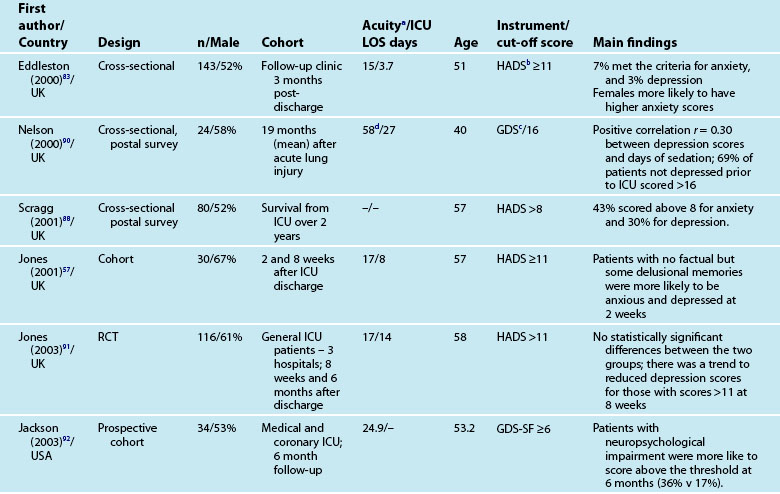
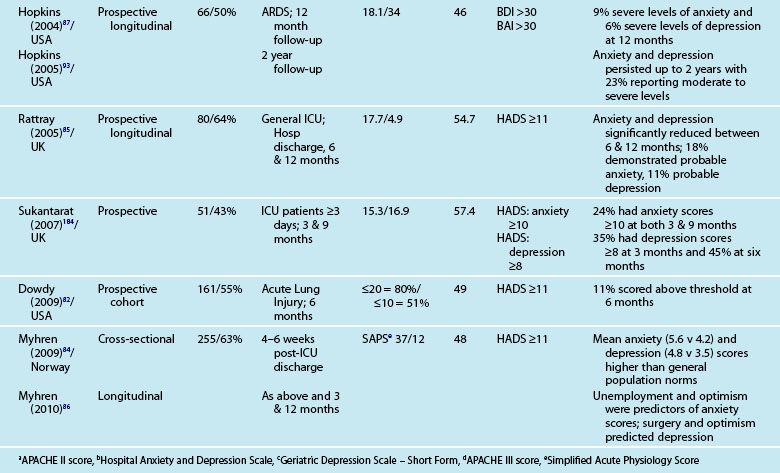
Reported prevalence of anxiety and depression after ICU discharge varies depending upon the questionnaire and ‘cut-off’ scores used, and the research design (see Table 4.4). For example, one study of an intensive care follow-up clinic reported anxiety prevalence of 7% three months after discharge;83 much less than a similar study where anxiety was 18% one year after discharge.85 Both studies used the HADS with scores of ≥11 to indicate an anxiety or depressive problem. Prevalence of depression in these studies was more equivalent, 10%83 and 11%.85 Table 4.4 provides a summary of studies reporting the prevalence of anxiety and depression. These differences may be explained by differences in case mix or timing of assessment.
Patients often exhibit high levels of distress at time of hospital discharge and these tend to reduce in the first year after discharge.85,90 However the episodic timing of assessments may not fully capture patterns of anxiety and depression, and establish whether full resolution is achieved. For example, in patients with ARDS, levels of depression increased from 16% at 1 year after discharge to 23% at 2 years.93 This may reflect prolonged recovery in general for this subgroup of patients, who tend to be among the most critically ill patients, with a mean ICU stay of 34 days noted. A rise in depression scores may therefore be a reflection of that prolonged physical recovery.
What is emerging from the literature is that certain patient demographic and clinical characteristics predict subsequent anxiety and depression, although not consistently. Women tend to be more anxious than men83,85 and younger patients more anxious than older patients.85 Other consequences of being in intensive care such as neuropsychological impairment can also predict significantly higher depression scores.92 Sicker patients, those with a longer length of ICU stay, and also a longer duration of sedation and mechanical ventilation are more likely to have measurable depression.82 This is perhaps not surprising as patients who are in intensive care longer tend to have more prolonged hospital stay and recovery period. What is also evident in the emerging literature is the effect of patients’ subjective intensive care experiences. These experiences tend to be reported as unpleasant memories of being in ICU16,57–59,84,85 and are discussed later in this chapter.
Depression is also associated with other aspects of recovery and in particular HRQOL. Depressed patients tend to rate their HRQOL as poorer than those who are not.85,93 However what is less clear is the direction of this relationship; it could be that patients with a poorer HRQOL tend to be depressed rather than depression leading to perceptions of poorer HRQOL. Patients who have psychological problems prior to intensive care are likely to develop these after discharge. Although assessment of pre-ICU status is difficult, in some cases this information can be obtained from relatives or caregivers.
Posttraumatic Stress
In recent years there has been increasing interest in the development of posttraumatic stress reactions such as Posttraumatic Stress Disorder (PTSD) as a response to critical illness,94,95 and there is increasing recognition of these symptoms as a problem for some intensive care survivors.14,72 Individuals do not perceive or respond to traumatic or life-threatening events in the same way, but there are commonalities96 including that events are often perceived as a threat to life, are uncontrollable and unpredictable97 and that they are beyond the usual human experience.98 Many symptoms of posttraumatic stress that patients experience in the initial days after intensive care discharge may be considered a normal reaction. Therefore practitioners need to clearly separate the normal from the abnormal response; this is achieved by assessing the severity, duration of symptoms, and their effect on an individual’s life. PTSD should not be diagnosed until at least one month after the event, and until the symptoms have been present for one month. Symptoms commonly cause problems in relation to work, social or other important activities;99 this is important to consider when developing critical care follow-up services. Importantly, PTSD symptoms may be reactivated after some time, and being in ICU may serve as a catalyst for some patients, e.g. reliving a war event.e.g.58
As with other psychological symptoms such as anxiety and depression, it has been difficult to establish the prevalence of PTSD after intensive care because of the use of self-report measures, different research designs, varied patient casemix and international variations in the delivery of intensive care. These variations have resulted in overestimation of the prevalence of PTSD and posttraumatic stress symptoms (PTSS),100 although note that patients with significant PTSS may be less likely to participate in research studies. While PTSD should be diagnosed through a structured clinical interview,12 few studies use this approach. One small study compared the prevalence of PTSD in patients who had daily sedation withdrawal versus those who did not; 6/19 patients who did not receive daily sedation withdrawal were diagnosed with PTSD, while 0/13 were diagnosed from the intervention group.68 The small sample size was a limitation but nonetheless these were important findings.
Patients may have significant PTSS without developing PTSD and it is mainly these symptoms that are assessed using the self-report measures. Reported prevalence of a significant posttraumatic stress reaction or PTSD is 14–27%.74 As for anxiety and depression, there are certain patient and clinical characteristics that can predict likelihood of a posttraumatic stress reaction. Trauma101 and younger patients tend to have higher scores on measures of posttraumatic stress.74,85,88 Aspects of an intensive care experience are associated also with a posttraumatic stress reaction. Patients with a longer ICU stay,85,90 longer duration of sedation and/or neuromuscular blockade,90 and mechanical ventilation74 are more likely to report posttraumatic stress symptoms. Patients who have daily sedative interruption had lower scores on the Impact of Event Scale.68 Importantly, daily sedative interruption or withdrawal, or titration of sedation is becoming more common in practice and therefore requires further research. Certain subgroups of patients appear to have a higher prevalence of PTSD (e.g. ARDS patients81), and PTSD can often endure for many years.
Memories and Perceptions
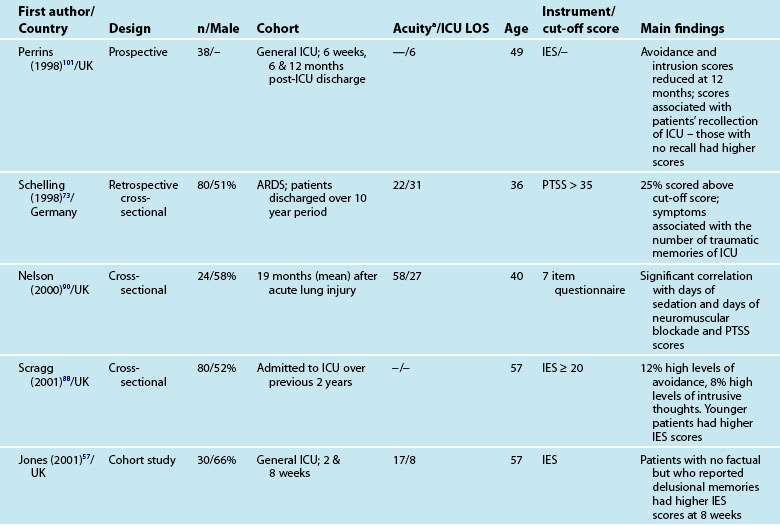
Interestingly, illness severity does not consistently predict a PTSS reaction,73,85 but rather perceptions of the intensive care experience. This is one of the unique features of being in intensive care; patients have little recall for factual events and often report large gaps where they remember very little about their critical illness. Patients’ accounts often include disturbing recollections with memories of ‘odd perceptual experiences’,54,102 ‘nightmares’ or ‘hallucinations’.57,58 While not all patients experience these, those who do so tend to report memories that are persecutory in nature,103 are often associated with feelings of being elsewhere,102 reliving a previous life event,104 or fighting for survival.102 These memories often seemed ‘real’ and were distressing to patients at the time, and may be recalled in detail some months afterwards.58 Having delusional rather than factual memories is more likely to result in distress;56,57,85,105 and lack of memory for factual events may result in longer-term psychological problems,57 with the important element being the content of the ICU memories rather than the number of memories. Table 4.5 summarises studies exploring posttraumatic stress after ICU.
Interventions to Improve Psychological Recovery
Although there is now strong empirical evidence that some patients experience significant psychological dysfunctions after a critical illness, it is less clear how to treat these symptoms. Systematic follow-up services may offer appropriate assessment support during recovery for individuals identified with psychological disturbances. Intensive care follow-up clinics where patients have the opportunity to discuss their intensive care experiences and receive information about what had happened to them could be a useful intervention, although there are currently no empirical data to support this,53 and further research work is required.
Patient diaries were also thought to be important in providing missing pieces of information that might help a patient make sense of their critical illness experience. A diary approach has been adopted in a number of European ICUs,106,107 and while there has been some variation in how the diaries were compiled and then viewed by a patient, there is emerging evidence that supports their use.108 Note however that not all patients may wish to be reminded of their ICU experience; this is especially the case for patients who demonstrate avoidant behaviours. Others may wish not to be reminded of being critically ill but wish to concentrate on recovery.53 Further research that incorporates these issues during assessment of posttraumatic stress symptoms will further establish the effectiveness of diary use (see Research Vignette later in this chapter).
The recent UK NICE guidelines109 emphasised regular assessment of patient recovery including psychological recovery. Assessment periods include during intensive care, ward-based care, before discharge home or community care and 2–3 months after ICU discharge, with the use of existing referral pathways and stepped care models to treat identified psychological dysfunctions. These services are usually well established and allow patients to be treated by appropriately qualified practitioners. The role of critical care practitioners may therefore be to establish the causes of psychological disturbances associated with critical illness, identifying at-risk patients through systematic and standardised screening activities, closely monitoring identified patients and referring to appropriate specialties where appropriate, to optimise their recovery trajectory while not introducing any further harm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree