R
Raynaud’s phenomenon
Description
Raynaud’s phenomenon is an episodic vasospastic disorder of the small cutaneous arteries, most frequently involving the fingers and toes. It occurs primarily in young women (typically between 15 and 40 years old). The pathogenesis is due to abnormalities in the vascular, intravascular, and neuronal mechanisms that cause an imbalance between vasodilation and vasoconstriction.
Raynaud’s phenomenon may occur in isolation or in association with an underlying disease (e.g., rheumatoid arthritis, scleroderma, or systemic lupus erythematosus). Other contributing factors include occupation-related conditions, such as use of vibrating machinery or work in cold environments, and exposure to heavy metals (e.g., lead).
Clinical manifestations
Exposure to cold, emotional upsets, tobacco use, and caffeine often bring on symptoms.
Nursing and collaborative management
When conservative management is ineffective, drug therapy is considered. Sustained-release calcium channel blockers (e.g., nifedipine [Procardia]) are the first-line drug therapy. Calcium channel blockers relax smooth muscles of the arterioles by blocking the influx of calcium into the cells. This reduces the frequency and severity of vasospastic attacks.
Prompt intervention is needed for patients with digital ulceration and/or critical ischemia. Treatment options include IV prostanoid therapy (e.g., iloprost), antibiotics, analgesics, and possibly an endothelin receptor antagonist (e.g., bosentan [Tracleer]) and surgical debridement of necrotic tissue. Sympathectomy is considered only in advanced cases.
 Patient and caregiver teaching
Patient and caregiver teaching
Teaching should be directed toward preventing recurrent episodes.
■ Temperature extremes should be avoided. Immersing hands in warm water often decreases the spasm.
■ Provide patients with information about stress management techniques as appropriate.
Reactive arthritis
Reactive arthritis (Reiter’s syndrome) is associated with a symptom complex that includes urethritis or cervicitis, conjunctivitis, and mucocutaneous lesions. It occurs more commonly in young men as compared with young women.
Individuals with inherited HLA-B27 are at increased risk of developing reactive arthritis after sexual contact or exposure to certain enteric pathogens, supporting the likelihood of a genetic predisposition.
Prognosis is favorable, with most patients recovering after 2 to 16 weeks. Because reactive arthritis is often associated with Chlamydia trachomatis infection, treatment of patients and their sexual partners with doxycycline (Vibramycin) is widely recommended. Drug therapy may also include nonsteroidal antiinflammatory drugs (NSAIDs), methotrexate, and sulfasalazine. Physical therapy may be helpful during disease recovery.
Refractive errors
Refractive errors are the most common visual problem. This defect prevents light rays from converging into a single focus on the retina. Defects are a result of corneal curvature irregularities, lens-focusing power, or eye length. Types of refractive errors include the following:
The major symptom of refractive errors is blurred vision. Additional complaints may include ocular discomfort, eye strain, or headaches. Management of refractive errors is correction, which may include eyeglasses, contact lenses, refractive surgery, or surgical implantation of an artificial lens.
Respiratory failure, acute
Description
The major function of the respiratory system is gas exchange, which involves the transfer of oxygen (O2) and carbon dioxide (CO2) between inhaled tidal volumes and circulating blood volume within the pulmonary capillary bed. Respiratory failure results when one or both of these gas-exchanging functions are inadequate.
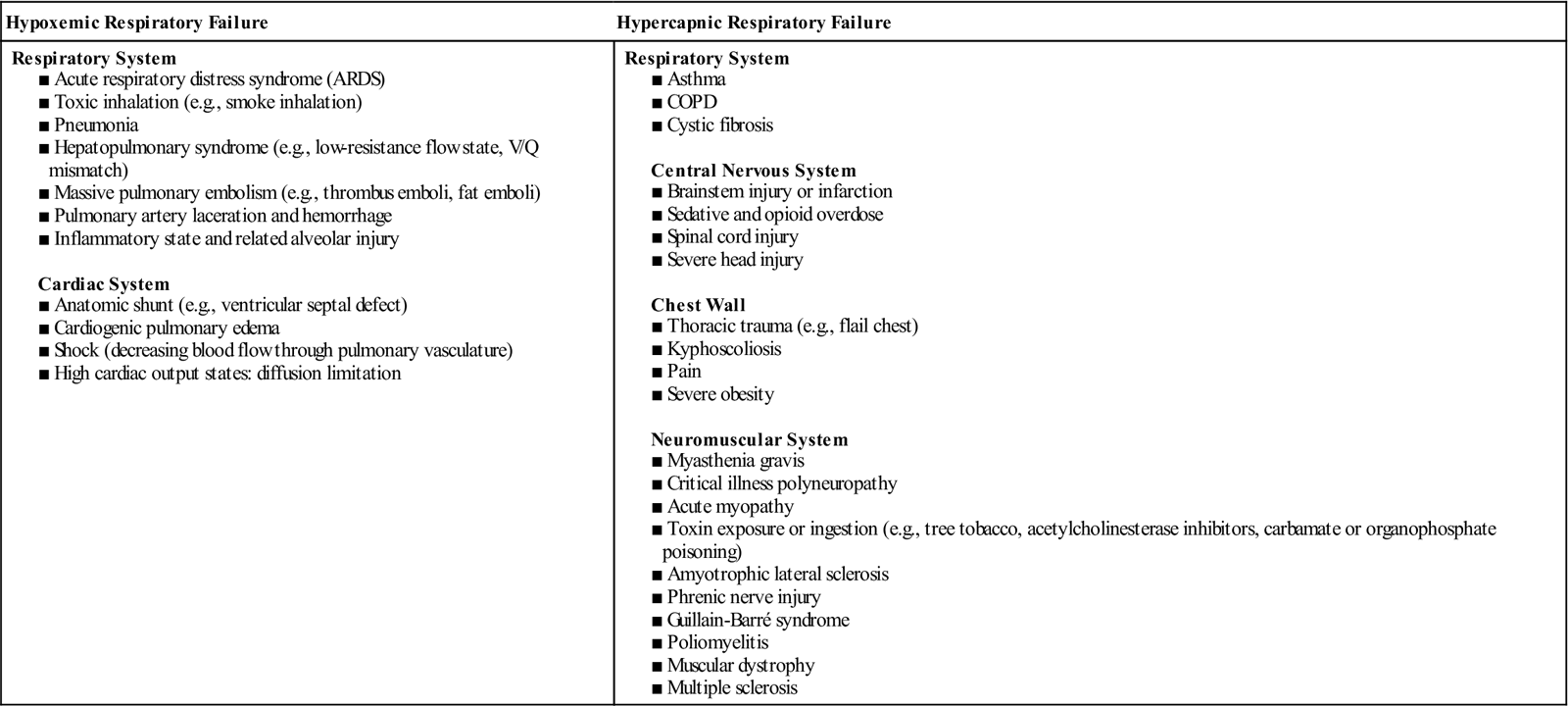
Respiratory failure is not a disease but a symptom of an underlying pathology affecting lung tissue function, O2 delivery, cardiac output (CO), or the baseline metabolic state. It is a condition that occurs because of one or more diseases involving the lungs or other body systems (Table 73). Respiratory failure is classified as hypoxemic or hypercapnic. Many patients experience both hypoxemic and hypercapnic respiratory failure.
Table 73
Causes of Hypoxemic and Hypercapnic Respiratory Failure*
Pathophysiology
Hypoxemic respiratory failure
Four physiologic mechanisms may cause hypoxemia and subsequent hypoxemic respiratory failure: (1) mismatch between ventilation (V) and perfusion (Q) commonly referred to as V/Q mismatch, (2) shunt, (3) diffusion limitation, and (4) hypoventilation. The most common causes are V/Q mismatch and shunt.
Frequently, hypoxemic respiratory failure is caused by a combination of V/Q mismatch, shunt, diffusion limitation, and alveolar hypoventilation.
Hypercapnic respiratory failure
Hypercapnic respiratory failure results from an imbalance between ventilatory supply and ventilatory demand. Normally, ventilatory supply far exceeds ventilatory demand. However, patients with preexisting lung disease such as severe COPD cannot effectively increase lung ventilation in response to exercise or metabolic demands. Hypercapnic respiratory failure is sometimes called ventilatory failure because the primary problem is the inability of the respiratory system to remove sufficient CO2 to maintain a normal PaCO2.
■ Many diseases can cause a limitation in ventilatory supply (see Table 73). They can be grouped into four categories: (1) abnormalities of the airways and alveoli, (2) abnormalities of the CNS, (3) abnormalities of the chest wall, and (4) neuromuscular conditions.
Clinical manifestations
Respiratory failure may develop suddenly (minutes or hours) or gradually (several days or longer). A sudden decrease in PaO2 or a rapid rise in PaCO2 implies a serious condition that can rapidly become a life-threatening emergency.
Manifestations are related to the extent of the change in PaO2 and PaCO2, the rapidity of change (acute versus chronic), and the ability to compensate to overcome this change. When the patient’s compensatory mechanisms fail, respiratory failure occurs. Because manifestations are variable, it is important to monitor trends in arterial blood gas (ABG) values and/or pulse oximetry to evaluate the extent of change.
The patient may have a rapid, shallow breathing pattern or a respiratory rate that is slower than normal. A change from a rapid to a slower rate in a patient in acute respiratory distress suggests extreme progression of respiratory fatigue and increased possibility of respiratory arrest.
Immediately report any change in mental status, such as agitation, confusion, or a decreased level of consciousness (LOC), because this change may indicate the onset of rapid deterioration and the need for mechanical ventilation.
Diagnostic studies
■ ABGs determine the levels of PaCO2, PaO2, bicarbonate, and pH.
■ Chest x-ray helps to identify possible causes of respiratory failure.
■ A catheter may be inserted into a peripheral artery for monitoring BP and obtaining ABGs.
■ Pulse oximetry monitors oxygenation status but reveals little about lung ventilation.
■ Other studies may include CBC, serum electrolytes, urinalysis, and ECG.
■ Sputum and blood cultures are obtained as necessary to determine sources of possible infection.
■ If pulmonary embolus is suspected, a V/Q lung scan or CT scan may be done.
In severe respiratory failure requiring endotracheal intubation, end-tidal CO2 (ETCO2) may be used to assess tube placement within the trachea immediately following intubation. ETCO2 may also be used during ventilator management to assess trends in lung ventilation. A central venous or pulmonary artery (PA) catheter is often used to measure hemodynamic parameters (e.g., central venous pressure, PA pressures, CO, pulmonary artery wedge pressure, central/mixed venous O2 saturation [ScvO2/SvO2]).
Nursing and collaborative management
Because many different problems can cause respiratory failure, specific care of these patients varies. Goals and related interventions to maximize O2 delivery are essential to improving the patient’s oxygenation and ventilation status. The primary goal is to treat the underlying cause of the respiratory failure. Other supportive goals include maintaining an adequate CO and hemoglobin concentration.



