3 Quality and Safety
After reading this chapter, you should be able to:
• describe the contribution that evidence-based nursing can make to critical care nursing practice.
• identify the steps in developing clinical practice guidelines.
• explain the role care bundles and checklists have in promoting quality and safety in critical care nursing practice.
• discuss rapid response systems used to respond to deteriorating patients.
• describe the use of information and communication technologies in critical care.
• identify techniques used to understand situations that place patients at risk of adverse events in critical care.
• identify strategies to improve the safety culture in critical care.
Introduction
Today’s critical care units are both busy and complex, where nurses, doctors and other health professionals use their knowledge, skills and technology to provide patient care. In fact, this complexity makes errors a common occurrence; one large international study in 205 Intensive Care Units (ICU) showed that 39 serious adverse events occurred per 100 patient days.1 The Institute of Medicine (IOM) in the USA defines quality of health care as ‘the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge’.2 Critical care nurses are well known for their skills in patient assessment. In fact, this ongoing surveillance of patient condition means that nurses are ideally positioned to prevent, discover and correct medical errors.3 Thus, nurses play a key role in improving quality and safety in health care. This chapter provides a review of quality and safety in critical care. First, an overview of evidence-based nursing and clinical practice guidelines is given to provide a foundation to consider quality and safety. Next, quality and quality monitoring is considered. Included in this section are the topics of care bundles, checklists, rapid response systems and information and communication technologies. Finally patient safety, including safety culture is described. In Chapter 2 we addressed risk management, clinical governance and the role of clinical leaders and managers in delivering critical care services; this information is complementary to what will be discussed in Chapter 3.
Evidence-Based Nursing
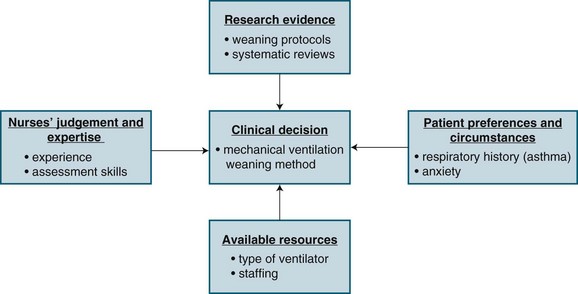
Evidence-based nursing (EBN) is the ‘Application of valid, relevant, research-based information in nurse decision-making.’4 Research evidence, however, is only one of four considerations in making a clinical decision. Three other considerations include: (1) knowledge of patients’ conditions (i.e. preferences and symptoms); (2) the nurses’ clinical expertise and judgment; and (3) the context in which the decision is taking place (i.e. setting, resources). Figure 3.1 provides a schematic representation of EBN, using an example of a decision about weaning a patient from a mechanical ventilator.
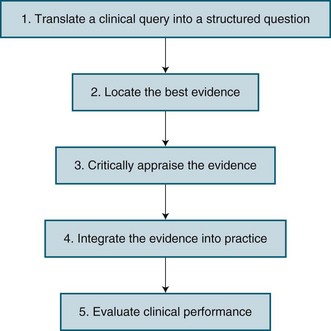
EBN has emerged as a way to improve nursing practice by considering the care that nurses give to patients, and whether this care results in the best possible outcomes for patients. It has been viewed as both an attitude and a process. As an attitude, it is a way of approaching practice that is critical and questioning. As a process, a number of steps in EBN have been described. Figure 3.2 identifies these steps, with more details about each step being provided below.
Translate a Clinical Query into a Structured Question
In situations where nurses have to make clinical decisions, it is important for them to carefully consider the issue or problem they are facing as it influences what research evidence should be used to make decisions. Thus, the first step in the EBN process is translating a clinical query into a well-defined, answerable, structured question. A well recognised approach is the Population, Intervention, Comparison, Outcome format, more often referred to as PICO. The Population reflects the patient group or clinical scenario of concern. The Intervention is one option for the particular nursing practice. The Comparison is the current practice, or the second option for practice. Finally, the Outcome is the effect that the nurse is hoping to achieve, which should reflect a patient outcome. Table 3.1 provides three examples of PICO questions relevant to critical care nursing.
Critically Appraise the Evidence
Once the various sources of evidence have been retrieved, they are then assessed for their quality and relevance to the clinical question. In Australia, the National Health and Medical Research Council (NHMRC)5 has described strategies to assess research evidence on the effectiveness of interventions. It provides a useful framework to consider research evidence for improving nursing interventions, and identifies three questions to ask regarding potential interventions:
This first question regarding the real effect relates to the strength of the research that has been conducted. The strength of the research has three dimensions: level of evidence, quality of the individual studies, and their statistical precision (denoted by P values or confidence intervals). Although there are a number of different evidence hierarchies (e.g. see Jennings & Loan, 2001),6 the framework used by the NHMRC5 is displayed in Table 3.2.
TABLE 3.2 NHMRC’s level of evidence5 designation for levels of evidence in the studies of effectiveness
| Level of evidence | Study design |
|---|---|
| I | Evidence obtained from a systematic review of all relevant randomised controlled studies |
| II | Evidence obtained from at least one properly designed randomised controlled trial |
| III-1 | Evidence obtained from well-designed pseudorandomised controlled trials (alternative allocation or some other method) |
| III-2 | Evidence obtained from comparative studies (including systematic reviews of such studies), with concurrent controls and allocation not randomised, cohort studies, case-control studies, or interrupted time series with a control group |
| III-3 | Evidence obtained from comparative studies with historical controls, two or more single-arm studies, or interrupted time series without a parallel control group |
| IV | Evidence obtained from case series, either post-test or pre-test/post-test |
The third question conveys the notion that potential benefits or outcomes of the intervention must be both important to the patient, and be able to be replicated in other settings. The NHMRC5 identifies three types of outcome: surrogate, clinical, and patient-relevant (which are not mutually exclusive) (see Table 3.3). Surrogate outcomes are often used in critical care where measurement of the actual physiological change (e.g. oxygen-carrying capacity of the blood) is replaced by a more accessible, and equally acceptable, parameter (e.g. oxygen saturation). Clinical outcomes are those of direct relevance to clinical practice, and patient-relevant outcomes are those likely to be articulated as significant by the patient/carer. When assessing research evidence, the type of outcome used in the research should be considered. Assessing the evidence results in an understanding of its quality of evidence for a particular nursing practice.
| Outcome | Definition | ICU example |
|---|---|---|
| Surrogate | Some physical sign or measurement substituted for a clinically meaningful outcome | |
| Clinical | Outcome defined on the basis of the problem | |
| Patient-relevant | Outcomes that are important to the patient |
Clinical Practice Guidelines
The development and use of clinical practice guidelines (CPGs) is one strategy to implement EBN. CPGs are statements about appropriate health care for specific clinical circumstances that assist practitioners in their day-to-day practice.7 They are systematically developed to assist clinicians, consumers and policy makers in healthcare decisions and provide critical summaries of available evidence on a particular topic.8 Other terms that are often used synonymously with CPGs include protocols and algorithms.
There are a number of benefits of using CPGs. They are seen to be central to quality patient care because, in essence, they standardise care.9 They can guide decisions and can be used to both justify and legitimise activities and practices.9 However, limitations have also been identified. Poorly developed guidelines may not improve care and may actually result in substandard care9. In the critical care area, the Intensive Care Coordination and Monitoring Unit of New South Wales Department of Health has led the development of CPGs associated with six common nursing interventions: (1) Eye care; (2) Oral care; (3) Suctioning a tracheal tube; (4) Endotracheal tube stabilisation; (5) Central line care; and (6) Arterial line care.10 Clinical audits are often used to establish the need to develop new protocols at the local unit level. Clinical audits generally involve chart reviews, but may also use direct observation or surveys of practice. Clinical audits often establish variation in practice without adequate justification.
Developing, Implementing and Evaluating Clinical Practice Guidelines
A number of steps are undertaken when developing clinical practice guidelines. Table 3.4 provides an overview of these steps, which has been adapted from Miller and Kearney’s work.9
TABLE 3.4 Steps in developing clinical practice guidelines9
| Step | Description |
|---|---|
| Find the evidence | After deciding on what is considered evidence, databases such as CINAHL and Medline must be searched to find relevant studies and expert opinions. |
| Evaluate the evidence | Relevant studies and expert opinion papers must be critically appraised for their strengths and weaknesses. This may or may not incorporate a systematic review. |
| Synthesise the evidence | General summary statements about the state of knowledge on a particular topic are developed. |
| Design the guidelines | Written summaries, algorithms and/or summary sheets will be developed that include statements about appropriate healthcare practices and their rationale. |
| Appraise the guidelines | Validity, reliability, clinical applicability, flexibility and clarity are some criteria that can be used to assess the guidelines. |
| Disseminate and implement the guidelines | Specific strategies such as seminars and patient chart reminders must be developed to increase awareness, acceptance and implementation of the guidelines. |
| Review and reassess the guidelines | Clinical audits and research may be used to regularly evaluate the impact the guidelines have had on patient care and outcomes. |
Based on the assessment of the CPG, revisions may be required. Next, strategies for disseminating and implementing the guidelines should be developed. Importantly, simply publishing and circulating CPGs will have a limited impact on clinical practice, so specific activities must be undertaken to promote CPG adherence. The following seven strategies have been shown to be moderately effective in promoting guideline adherence: (1) interactive small group sessions; (2) educational outreach visits; (3) reminders; (4) computerised decision support; (5) introduction of computers in practice; (6) mass media campaigns; and (7) combined interventions.7 Finally, a process for regularly evaluating and updating the guidelines must be developed, which may involve quality improvement activities or clinical research. In summary, by developing, using and evaluating clinical practice guidelines, nurses may improve patient care and outcomes. Additionally, use of CPGs should ensure that nursing practice is based on the best available evidence.
Quality and Safety Monitoring
This section discusses unit-level measures used to evaluate the quality and safety of care for critically ill patients. Quality and safety in healthcare is commonly described in terms of Donabedian’s approach11 with three major domains:
1. Patient outcomes – the results of care in terms of recovery, restoration of function and/or survival (e.g. mortality, health-related quality of life).
2. Process – the practices involved in the delivery of care (e.g. pressure ulcer prevention strategies).
3. Structure – the way the healthcare setting and/or system is organised to deliver care (e.g. staffing, beds, equipment).
More recently, a fourth domain of culture or context has been suggested specifically for patient safety models to evaluate the context in which care is delivered.12 The contemporary model for healthcare improvement recognises that the resources (structure) and activities carried out (processes) must be addressed within a given context (culture) to improve the quality of care (outcome). The overall aim of quality improvement (QI) is to provide safe, effective, patient-centred, timely, efficient and equitable health care.13 QI activities identify and address gaps between knowledge and practice. Importantly, these activities need to reflect the most recent and robust clinical evidence to improve patient care and reduce harm. The most common approach used for rapid improvement in healthcare is the plan–do–study–act (PDSA)14 method where four essential steps are carried out in a continuous fashion to ensure processes are continually improved:
1. Plan – identify a goal, specify aims and objectives to improving an area of clinical practice, and how that might be achieved (i.e. how to test the intervention).
2. Do – implement the plan of action, collect relevant information that will inform whether the intervention was successful and in what way, taking note of problems and unexpected observations that arise.
3. Study – the results of the intervention, particularly its impact on practice improvement, noting any strengths and limitations of the intervention.
4. Act – determine whether the intervention should be adopted, abandoned or adapted for further rapid cycle testing recommencing at the Plan phase.
A variety of specific activities have been used in the ICU setting to translate findings from the literature to improve clinical practice.15 Quality monitoring includes measurement of, and response to, the incidence and patterns of adverse events (AEs). Adverse events occur in up to 17% of all hospital admissions,13 and cost the Australian healthcare system an estimated $2 billion per year.16 About 18,000 hospital deaths per year are associated with AEs13 and generally occur as a result of system errors. Half the AEs were deemed preventable with such strategies as improved protocols, better-quality monitoring, enhanced training and opportunities to consult with specialists or peers on clinical decisions.17 Studies have identified specific contributing factors for adverse events related to patient airway18 and intra-hospital transports.19
A number of methods for reporting AE such as direct observation chart audit and self or facilitated reporting can be used; each has its strengths and limitations. Trained observers report more unintended events but this method is expensive, labour intensive and vulnerable to the Hawthorne effect.20 Both chart audits and incident reporting only reflect what is charted or reported, but even when chart audit, incident reporting, general practitioner reporting and external sources, such as coronial review, are used together, some adverse events will be missed.21 Importantly, self or facilitated reporting, such as the Australian Incident Monitoring Study (AIMS)22,23 are routinely used surveillance methods in many countries.
Medication administration is the most common intervention in health care, but the medication management process in the acute hospital setting is complex, and creates risk for patients. As a result, medication-related events are the commonest AE for hospitalised patients.24 Adverse drug events (ADEs) are common in Australian hospitals, with preventable, high-impact events involving anticoagulants, anti-inflammatories and cardiovascular drugs (over 50% of ADEs), as well as antineoplastics, opioids, steroids and antibiotics (commonly used in critical care units).17 Events are clinically significant in 20% of cases.17 A number of strategies have been instituted in Australia under the auspices of the National Medicines Policy, including the quality use of medicines (QUM) framework. There, however, remains a lack of consensus on how to measure medication safety25 – either by error or adverse event – where:
The actual incidence of both measures is higher than what is reported.17,26 Fortunately, most healthcare errors do not result in patient harm because of safety-net processes.24 Despite this, it has been estimated that one potentially serious intravenous drug error occurs every day in a 400-bed hospital.27 Approximately 5% of medication errors relate to infusion pumps. These pumps are used to administer high-impact medications, such as inotropes, heparin or antineoplastics.28 It is therefore important to evaluate interventions that can reduce the incidence and impact of adverse intravenous drug events, particularly in critical care settings.29,30 Recent evidence suggests that nurses who are interrupted whilst administering medications may have an increased risk of making medication errors,31 prompting calls for all healthcare workers to make concerted efforts to reduce interruptions to clinical tasks.32 Other activities examining quality of care include the analysis of incident reports such as the Australian Incident Monitoring Study (AIMS),22,23 Quality in Australian Health Care Study (QAHCS)17 and the Australian Council on Healthcare Standards (ACHS) indicators.33 Current ACHS indicators for intensive care include:
• inability to admit a patient to the ICU due to inadequate resources
• elective surgery deferred or cancelled due to lack of ICU/HDU bed
• patients transferred to another facility due to unavailability of an ICU bed
• delays on discharging patients from the ICU of more than 12 hours
• patients discharged from the ICU after hours (i.e. between 6pm and 6am)
• recognising and responding to clinical deterioration within 72 hours of being discharged from ICU
• patients being treated appropriately for VTE prophylaxis within 24 hours of admission to the ICU
• ICU central line-associated bacteraemia rates
• use of patient assessment systems (participation in national databases and surveys).33
Similar activities are evident internationally, where concepts of ‘safety science’ (error reduction and recovery) are being applied to critical care practice.29,34–36 Process indicators of quality care have been developed, including care related to the prevention of ventilator-associated pneumonia (VAP) and central venous catheter management. Table 3.5 outlines process indicators with good clinical evidence and/or strong recommendations for use by professional bodies, such as the Agency for Healthcare Research and Quality (AHRQ) in the USA.
TABLE 3.5 Evidence based process indicators
| Process | Process indicator |
|---|---|
| 1. Central venous catheter management | Maximum sterile barriers Real-time ultrasound guidance during insertion Antibiotic-impregnated catheter |
| 2. Prevention of ventilator-associated pneumonia | Elevated head of bed Continuous aspiration of subglottic secretions Stress ulcer prophylaxis |
| 3. Reducing mechanical ventilation | Low tidal volumes for acute respiratory distress syndrome Weaning protocols Sedation protocols Appropriate use of analgesia and sedation |
| 4. Pressure ulcer prevention | Use of pressure-relieving materials |
A range of clinical support tools have been developed and are used to measure compliance with these best practice clinical standards. Daily goals forms, for example, have been used to aid communication between clinicians during and after multidisciplinary ward rounds and ensure that all staff are aware of what care the patient should be receiving and what the clinical plan is.37,38 A popular mnemonic developed for use by ICU clinicians during patient assessment is the ‘FASTHUG’ which stands for Feeding, Analgesia, Sedation, Thromboembolism prophylaxis, Head-of-bed elevation, stress ulcer prevention and Glucose management.39 Along with care bundles and checklists (detailed below) these tools facilitate standardised care and improve communication between clinicians.38
Care Bundles
An evolving QI approach to the optimal use of best practice guidelines at the bedside is the development of ‘care bundles’. A care bundle is a set of evidence-based interventions or processes of care, applied to selected patients. A number of bundles have been developed for critical care by the Institute for Healthcare Improvement (IHI) in the USA (see Table 3.6).
TABLE 3.6 Institute for Healthcare improvement care bundles
| Bundle name | Aim | Bundle components |
|---|---|---|
| Central line | Prevent central-line associated bacteraemia | |
| Ventilator care | Prevent ventilator-associated pneumonia | |
| Sepsis resuscitation | Reduce mortality due to severe sepsis | |
| Sepsis management | Reduce mortality due to severe sepsis |
Table 3.7 outlines studies examining the process of care delivery in critical care units, including those where care bundles were implemented and evaluated. Increased bundle compliance was associated with decreased ICU length of stay (LOS), reduced ventilator days and increased ICU patient throughput,40 and decreased rates of ventilator-associated pneumonia.41 Other quality improvement studies targeted similar processes of care without taking the bundled approach. A range of measures demonstrated improved outcomes:
• decreased VAP,42,43 catheter-related bloodstream infection (CR-BSI) rates and LOS43
• increased days between CR-BSIs44
• decreased hospital mortality as the number of process interventions increased45
• reduction in severity-adjusted total hospital costs related to improvements in process measures of care, including glucose control, use of enteral feeding and appropriate sedation.46
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree