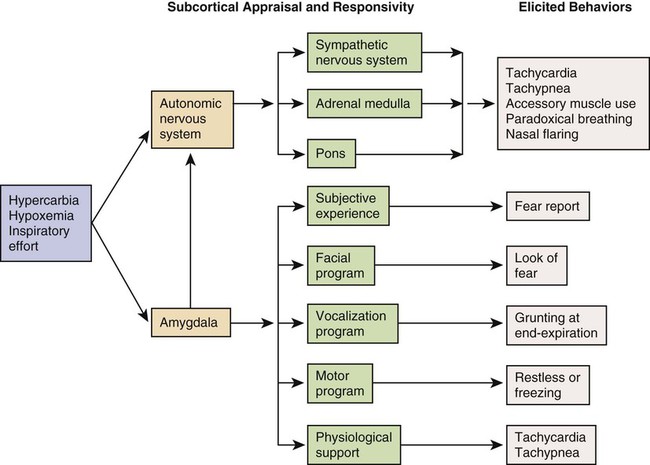
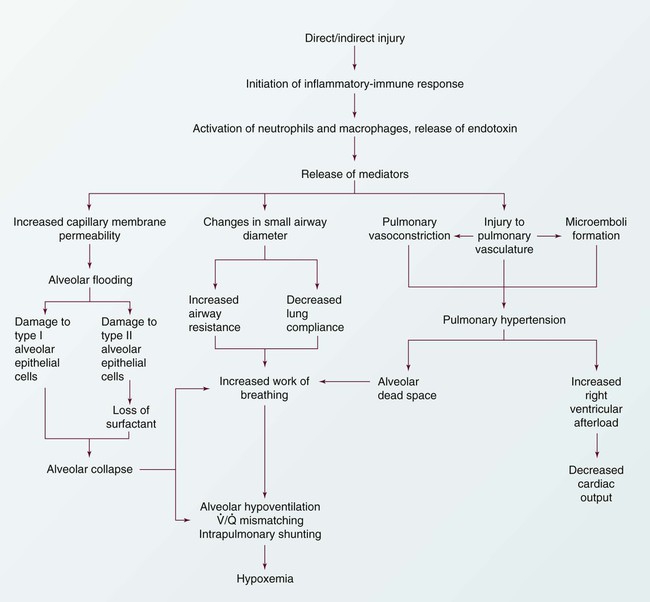
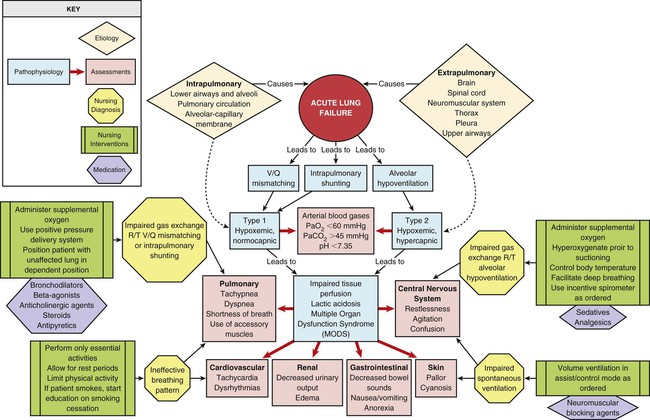
Chapter 20 Understanding the pathology of the disease, the areas of assessment on which to focus, and the usual medical management allows the critical care nurse to more accurately anticipate and plan nursing interventions. This chapter focuses on pulmonary disorders commonly seen in the critical care environment. Acute lung failure (ALF),1 also known as acute respiratory failure, is a clinical condition in which the pulmonary system fails to maintain adequate gas exchange.1,2 It is the most common type of organ failure seen in the critical care unit, with approximately 56% of the patients in the critical care unit experiencing it.1 The mortality rate for patients with ALF is between 30% to 40%, with more than one third of patients not surviving to discharge.3 ALF results from a deficiency in the performance of the pulmonary system (Fig. 20-1).2,4 It usually occurs secondary to another disorder that has altered the normal function of the pulmonary system in such a way as to decrease the ventilatory drive, decrease muscle strength, decrease chest wall elasticity, decrease the lung’s capacity for gas exchange, increase airway resistance, or increase metabolic oxygen requirements.1,5 ALF can be classified as hypoxemic normocapnic respiratory failure (type I) or hypoxemic hypercapnic respiratory failure (type II), depending on analysis of the patient’s arterial blood gases (ABGs). In type I respiratory failure, the patient presents with a low Pao2 and a normal Paco2, whereas in type II respiratory failure, Pao2 is low and Paco2 is high.2,4 The etiologies of ALF may be classified as extrapulmonary or intrapulmonary, depending on the component of the respiratory system that is affected. Extrapulmonary causes include disorders that affect the brain, the spinal cord, the neuromuscular system, the thorax, the pleura, and the upper airways. Intrapulmonary causes include disorders that affect the lower airways and alveoli, the pulmonary circulation, and the alveolar-capillary membrane.1,6 Table 20-1 lists the different etiologies of ALF and their associated disorders. TABLE 20-1 ETIOLOGIES OF ACUTE LUNG FAILURE Hypoxemia is the result of impaired gas exchange and is the hallmark of ALF. Hypercapnia may be present, depending on the underlying cause of the problem. The main causes of hypoxemia are alveolar hypoventilation, ventilation/perfusion (V/Q) mismatching, and intrapulmonary shunting.1,2,7 Type I respiratory failure usually results from V/Q mismatching and intrapulmonary shunting, whereas type II respiratory failure usually results from alveolar hypoventilation, which may or may not be accompanied by V/Q mismatching and intrapulmonary shunting.2 Alveolar hypoventilation occurs when the amount of oxygen being brought into the alveoli is insufficient to meet the metabolic needs of the body.6 This can be the result of increasing metabolic oxygen needs or decreasing ventilation.5 Hypoxemia caused by alveolar hypoventilation is associated with hypercapnia and commonly results from extrapulmonary disorders.1,2,7 V/Q mismatching occurs when ventilation and blood flow are mismatched in various regions of the lung in excess of what is normal. Blood passes through alveoli that are underventilated for the given amount of perfusion, leaving these areas with a lower-than-normal amount of oxygen. V/Q mismatching is the most common cause of hypoxemia and is usually the result of alveoli that are partially collapsed or partially filled with fluid.1,2,7 The extreme form of V/Q mismatching, intrapulmonary shunting, occurs when blood reaches the arterial system without participating in gas exchange. The mixing of unoxygenated (shunted) blood and oxygenated blood lowers the average level of oxygen present in the blood. Intrapulmonary shunting occurs when blood passes through a portion of a lung that is not ventilated. This may be the result of 1) alveolar collapse secondary to atelectasis; or 2) alveolar flooding with pus, blood, or fluid.1,2,7 If allowed to progress, hypoxemia can result in a deficit of oxygen at the cellular level. As the tissue demands for oxygen continue and the supply diminishes, an oxygen supply/demand imbalance occurs and tissue hypoxia develops. Decreased oxygen to the cells contributes to impaired tissue perfusion and the development of lactic acidosis and multiple organ dysfunction syndrome.8 The patient with ALF may experience a variety of clinical manifestations, depending on the underlying cause and the extent of tissue hypoxia. The clinical manifestations commonly seen in the patient with ALF are usually related to the development of hypoxemia, hypercapnia, and acidosis (Fig. 20-2).9 Because the clinical symptoms are so varied, they are not considered reliable in predicting the degree of hypoxemia or hypercapnia2 or the severity of ALF. Diagnosing and following the course of respiratory failure is best accomplished by ABG analysis. ABG analysis confirms the level of Paco2, Pao2, and blood pH. ALF is generally accepted as being present when the Pao2 is less than 60 mm Hg. If the patient is also experiencing hypercapnia, the Paco2 will be greater than 45 mm Hg. In patients with chronically elevated Paco2 levels, these criteria must be broadened to include a pH less than 7.35.9 A variety of additional tests are performed depending on the patient’s underlying condition. These include bronchoscopy for airway surveillance or specimen retrieval, chest radiography, thoracic ultrasound, thoracic computed tomography (CT), and selected lung function studies.10 Medical management of the patient with ALF is aimed at treating the underlying cause, promoting adequate gas exchange, correcting acidosis, initiating nutrition support, and preventing complications. Medical interventions to promote gas exchange are aimed at improving oxygenation and ventilation.1 Actions to improve oxygenation include supplemental oxygen administration, either with a low flow system or a high flow system,11 and the use of positive airway pressure.12 The purpose of oxygen therapy is to correct hypoxemia, and although the absolute level of hypoxemia varies in each patient, most treatment approaches aim to keep the arterial hemoglobin oxygen saturation greater than 90%.9 The goal is to keep the tissues’ needs satisfied but not produce hypercapnia or oxygen toxicity.9 Supplemental oxygen administration is effective in treating hypoxemia related to alveolar hypoventilation and V/Q mismatching. When intrapulmonary shunting exists, supplemental oxygen alone is ineffective.11 In this situation, positive pressure is necessary to open collapsed alveoli and facilitate their participation in gas exchange. Positive pressure is delivered via invasive and noninvasive mechanical ventilation. To avoid intubation, positive pressure is usually administered initially noninvasively via a mask.13 For further information on supplemental oxygen therapy and noninvasive ventilation, see Chapter 21. Interventions to improve ventilation include the use of noninvasive and invasive mechanical ventilation. Depending on the underlying cause and the severity of the ALF, the patient may be treated initially with noninvasive ventilation.13 However, one study found that those patients with a pH of less than 7.25 at initial presentation had an increased likelihood of the need for invasive mechanical ventilation.14 The selection of ventilatory mode and settings depends on the patient’s underlying condition, severity of respiratory failure, and body size. Initially the patient is started on volume ventilation in the assist/control mode. In the patient with chronic hypercapnia, the settings should be adjusted to keep the ABG values within the parameters expected to be maintained by the patient after extubation.15 For further information on mechanical ventilation see Chapter 21. Medications to facilitate dilation of the airways may also be of benefit in the treatment of the patient with ALF. Bronchodilators, such as beta2-agonists and anticholinergic agents, aid in smooth muscle relaxation and are of particular benefit to patients with airflow limitations. Methylxanthines, such as aminophylline, are no longer recommended because of their negative side effects. Steroids also are often administered to decrease airway inflammation and enhance the effects of the beta2-agonists. Mucolytics and expectorants are also no longer used since they have been found to be of no benefit in this patient population.16 Sedation is necessary in many patients to assist with maintaining adequate ventilation. It can be used to comfort the patient and decrease the work of breathing, particularly if the patient is fighting the ventilator. Analgesics should be administered for pain control.17,18 In some patients, sedation does not decrease spontaneous respiratory efforts enough to allow adequate ventilation. Neuromuscular paralysis may be necessary to facilitate optimal ventilation. Paralysis also may be necessary to decrease oxygen consumption in the severely compromised patient.18 Acidosis may occur in the patient for a number of reasons. Hypoxemia causes impaired tissue perfusion, which leads to the production of lactic acid and the development of metabolic acidosis. Impaired ventilation leads to the accumulation of carbon dioxide and the development of respiratory acidosis. Once the patient is adequately oxygenated and ventilated, the acidosis should correct itself. The use of sodium bicarbonate to correct the acidosis has been shown to be of minimal benefit to the patient and thus is no longer recommended as first-line treatment. Bicarbonate therapy shifts the oxygen-hemoglobin dissociation curve to the left and can worsen tissue hypoxia. Sodium bicarbonate may be used if the acidosis is severe (pH less than 7.1), refractory to therapy, and causing dysrhythmias or hemodynamic instability.19 The initiation of nutrition support is of utmost importance in the management of the patient with ALF. The goals of nutrition support are to meet the overall nutritional needs of the patient while avoiding overfeeding, to prevent nutrition delivery-related complications, and to improve patient outcomes.20 Failure to provide the patient with adequate nutrition support results in the development of malnutrition. Both malnutrition and overfeeding can interfere with the performance of the pulmonary system, further perpetuating ALF. Malnutrition decreases the patient’s ventilatory drive and muscle strength, whereas overfeeding increases carbon dioxide production, which then increases the patient’s ventilatory demand, resulting in respiratory muscle fatigue.21 The enteral route is the preferred method of nutrition administration. If the patient cannot tolerate enteral feedings or cannot receive enough nutrients enterally, he or she will be started on parenteral nutrition. Because the parenteral route is associated with a higher rate of complications, the goal is to switch to enteral feedings as soon as the patient can tolerate them.20,21 Nutrition support should be initiated before the third day of mechanical ventilation for the well-nourished patient and within 24 hours for the malnourished patient.20,21 The patient with ALF may experience a number of complications including ischemic-anoxic encephalopathy,22 cardiac dysrhythmias,23 venous thromboembolism (VTE),24 and gastrointestinal bleeding.25 Ischemic-anoxic encephalopathy results from hypoxemia, hypercapnia, and acidosis.22 Dysrhythmias are precipitated by hypoxemia, acidosis, electrolyte imbalances, and the administration of beta2-agonists.23 Maintaining oxygenation, normalizing electrolytes, and monitoring medication levels will facilitate the prevention and treatment of encephalopathy and dysrhythmias.22,23 VTE is precipitated by venous stasis resulting from immobility and can be prevented through the use of intermittent pneumatic compression devices and low-dose unfractionated heparin or low–molecular-weight heparin (LMWH).24 Gastrointestinal bleeding can be prevented through the use of histamine receptor antagonists, cytoprotective agents, or proton pump inhibitors.25 In addition, the patient is at risk for the complications associated with an artificial airway, mechanical ventilation, enteral and parenteral nutrition, and peripheral arterial cannulation. Nursing management of the patient with ALF incorporates a variety of nursing diagnoses (Box 20-1). Nursing care is directed by the specific cause of the respiratory failure, although some common interventions are used. The nurse has a significant role in optimizing oxygenation and ventilation, providing comfort and emotional support, maintaining surveillance for complications, and educating the patient and family. Nursing interventions to optimize oxygenation and ventilation include positioning, preventing desaturation, and promoting secretion clearance. Positioning of the patient with ALF depends on the type of lung injury and the underlying cause of hypoxemia. For those patients with V/Q mismatching, positioning is used to facilitate better matching of ventilation with perfusion to optimize gas exchange.26 Because gravity normally facilitates preferential ventilation and perfusion to the dependent areas of the lungs, the best gas exchange would take place in the dependent areas of the lungs.11 Thus the goal of positioning is to place the least affected area of the patient’s lung in the most dependent position. Patients with unilateral lung disease should be positioned with the healthy lung in a dependent position.26,27 Patients with diffuse lung disease may benefit from being positioned with the right lung down, because it is larger and more vascular than the left lung.27,28 For those patients with alveolar hypoventilation, the goal of positioning is to facilitate ventilation. These patients benefit from nonrecumbent positions such as sitting or a semierect position.29 In addition, semirecumbency has been shown to decrease the risk of aspiration and inhibit the development of hospital-associated pneumonia.30 Frequent repositioning (at least every 2 hours) is beneficial in optimizing the patient’s ventilatory pattern and V/Q matching.31 A number of activities can prevent desaturation from occurring. These include performing procedures only as needed, hyperoxygenating the patient before suctioning, providing adequate rest and recovery time between various procedures, and minimizing oxygen consumption. Interventions to minimize oxygen consumption include limiting the patient’s physical activity, administering sedation to control anxiety, and providing measures to control fever.29 The patient should be continuously monitored with a pulse oximeter to warn of signs of desaturation. Interventions to promote secretion clearance include providing adequate systemic hydration, humidifying supplemental oxygen, coughing, and suctioning. Postural drainage and chest percussion and vibration have been found to be of little benefit in the critically ill patient32,33 and thus are not discussed here. To facilitate deep breathing, the patient’s thorax should be maintained in alignment and the head of the bed elevated 30 to 45 degrees. This position best accommodates diaphragmatic descent and intercostal muscle action. Once the patient is extubated, deep breathing and incentive spirometry should be started as soon as possible. Deep breathing involves having the patient take a deep breath and holding it for approximately 3 seconds or longer. Incentive spirometry involves having the patient take at least 10 deep, effective breaths per hour using an incentive spirometer. These actions help prevent atelectasis and re-expand any collapsed lung tissue. The chest should be auscultated during inflation to ensure that all dependent parts of the lung are well ventilated and to help the patient understand the depth of breath necessary for optimal effect. Coughing should be avoided unless secretions are present because it promotes collapse of the smaller airways. Early in the patient’s hospital stay, the patient and family should be taught about ALF, its etiologies, and its treatment. As the patient moves toward discharge, teaching should focus on the interventions necessary for preventing the reoccurrence of the precipitating disorder (Box 20-2). If the patient smokes, he or she should be encouraged to stop smoking and be referred to a smoking cessation program (Box 20-3). In addition, the importance of participating in a pulmonary rehabilitation program should be stressed. Collaborative management of the patient with ALF is outlined in Box 20-4. Acute respiratory distress syndrome (ARDS) is a systemic process that is considered to be the pulmonary manifestation of multiple organ dysfunction syndrome.34 It is characterized by noncardiac pulmonary edema and disruption of the alveolar-capillary membrane as a result of injury to either the pulmonary vasculature or the airways.35 Many different diagnostic criteria have been used to identify ARDS, which has led to confusion, particularly among researchers. In 2012, in an attempt to address the limitations of the existing definition of ARDS, the ARDS Definition Task Force drafted a new definition (known as the Berlin Definition) of ARDS. This definition eliminated the term “acute lung injury” and proposed three distinct categories (mild, moderate, and severe) of ARDS based on the severity of hypoxemia. The Berlin Definition of ARDS is as follows: • Timing—within 1 week of known clinical insult or new or worsening respiratory symptoms • Chest imaging—bilateral opacities not fully explained by effusions, lobar/lung collapse or nodules • Origin of edema—respiratory failure not fully explained by heart failure or fluid overload; need objective assessment to exclude hydrostatic edema if no risk factor present • Oxygenation—mild (200 mg Hg less than Pao2/Fio2 less than or equal to 300 mm Hg with positive end-respiratory airway pressure [PEEP] or constant positive airway pressure [CPAP] greater than or equal to 5 cm H2O); Moderate (100 mg Hg less than Pao2/Fio2 less than or equal to 200 mm Hg with PEEP greater than or equal to 5 cm H2O); or Severe (Pao2/Fio2 less than or equal to 100 mm Hg with PEEP greater than or equal to 5 cm H2O).36 The mortality rate for ARDS is estimated to be 34% to 58%.37 A wide variety of clinical conditions is associated with the development of ARDS. These are categorized as direct or indirect, depending on the primary site of injury (Box 20-5).35,38 Direct injuries are those in which the lung epithelium sustains a direct insult. Indirect injuries are those in which the insult occurs elsewhere in the body and mediators are transmitted via the bloodstream to the lungs. Sepsis, aspiration of gastric contents, diffuse pneumonia, and trauma were found to be major risk factors for the development of ARDS.37 The progression of ARDS can be described in three phases: exudative, fibroproliferative, and resolution. ARDS is initiated with stimulation of the inflammatory-immune system as a result of a direct or indirect injury (Fig. 20-3). Inflammatory mediators are released from the site of injury, resulting in the activation and accumulation of the neutrophils, macrophages, and platelets in the pulmonary capillaries. These cellular mediators initiate the release of humoral mediators that cause damage to the alveolar-capillary membrane.38 Within the first 72 hours after the initial insult, the exudative phase or acute phase ensues. Once released, the mediators cause injury to the pulmonary capillaries, resulting in increased capillary membrane permeability leading to the leakage of fluid filled with protein, blood cells, fibrin, and activated cellular and humoral mediators into the pulmonary interstitium. Damage to the pulmonary capillaries also causes the development of microthrombi and elevation of pulmonary artery pressures. As fluid enters the pulmonary interstitium, the lymphatics are overwhelmed and unable to drain all the accumulating fluid, resulting in the development of interstitial edema. Fluid is then forced from the interstitial space into the alveoli, resulting in alveolar edema. Pulmonary interstitial edema also causes compression of the alveoli and small airways. Alveolar edema causes swelling of the type I alveolar epithelial cells and flooding of the alveoli. Protein and fibrin in the edema fluid precipitate the formation of hyaline membranes over the alveoli. Eventually, the type II alveolar epithelial cells are also damaged, leading to impaired surfactant production. Injury to the alveolar epithelial cells and the loss of surfactant lead to further alveolar collapse.38,39 Hypoxemia occurs as a result of intrapulmonary shunting and V/Q mismatching secondary to compression, collapse, and flooding of the alveoli and small airways. Increased work of breathing occurs as a result of increased airway resistance, decreased functional residual capacity (FRC), and decreased lung compliance secondary to atelectasis and compression of the small airways. Hypoxemia and the increased work of breathing lead to patient fatigue and the development of alveolar hypoventilation. Pulmonary hypertension occurs as a result of damage to the pulmonary capillaries, microthrombi, and hypoxic vasoconstriction leading to the development of increased alveolar dead space and right ventricular afterload. Hypoxemia worsens as a result of alveolar hypoventilation and increased alveolar dead space. Right ventricular afterload increases and leads to right ventricular dysfunction and a decrease in cardiac output (CO).38 This phase begins as disordered healing and starts in the lungs. Cellular granulation and collagen deposition occur within the alveolar-capillary membrane. The alveoli become enlarged and irregularly shaped (fibrotic) and the pulmonary capillaries become scarred and obliterated. This leads to further stiffening of the lungs, increasing pulmonary hypertension, and continued hypoxemia.38,39 Recovery occurs over several weeks as structural and vascular remodeling take place to re-establish the alveolar-capillary membrane. The hyaline membranes are cleared and intra-alveolar fluid is transported out of the alveolus into the interstitium. The type II alveolar epithelial cells multiply, some of which differentiate to type I alveolar epithelial cells, facilitating the restoration of the alveolus. Alveolar macrophages remove cellular debris.38,39 Initially the patient with ARDS maybe seen with a variety of clinical manifestations, depending on the precipitating event. As the disorder progresses, the patient’s signs and symptoms can be associated with the phase of ARDS that he or she is experiencing (Table 20-2). During the exudative phase, the patient presents with tachypnea, restlessness, apprehension, and moderate increase in accessory muscle use. During the fibroproliferative phase, the patient’s signs and symptoms progress to agitation, dyspnea, fatigue, excessive accessory muscle use, and fine crackles as respiratory failure develops.40,41 TABLE 20-2 PHYSIOLOGY AND ASSOCIATED PHYSICAL EXAMINATION OF PATIENT WITH ARDS Modified from Phillips JK. Management of patients with acute respiratory distress syndrome. Crit Care Nurs Clin North Am.1999;11(2):233. Arterial blood-gas analysis reveals a low Pao2, despite increases in supplemental oxygen administration (refractory hypoxemia).40 Initially the Paco2 is low as a result of hyperventilation, but eventually the Paco2 increases as the patient fatigues. The pH is high initially but decreases as respiratory acidosis develops.40,41 Initially the chest x-ray film may be normal, because changes in the lungs do not become evident for up to 24 hours. As the pulmonary edema becomes apparent, diffuse, patchy interstitial and alveolar infiltrates appear. This progresses to multifocal consolidation of the lungs, which appears as a “whiteout” on the chest x-ray film.40 Medical management of the patient with ARDS involves a multifaceted approach. This strategy includes treating the underlying cause, promoting gas exchange, supporting tissue oxygenation, and preventing complications. Given the severity of hypoxemia, the patient is intubated and mechanically ventilated to facilitate adequate gas exchange.42 Traditionally the patient with ARDS was ventilated with a mode of volume ventilation, such as assist/control ventilation (A/CV) or synchronized intermittent mandatory ventilation (SIMV), with tidal volumes adjusted to deliver 10 to 15 mL/kg. Current research now indicates that this approach may have actually led to further lung injury. It is now known that repeated opening and closing of the alveoli cause injury to the lung units (atelectrauma), resulting in inhibited surfactant production, and increased inflammation (biotrauma), resulting in the release of mediators and an increase in pulmonary capillary membrane permeability. In addition, excessive pressure in the alveoli (barotrauma) or excessive volume in the alveoli (volutrauma) leads to excessive alveolar wall stress and damage to the alveolar-capillary membrane, resulting in air escaping into the surrounding spaces.42 Thus several different approaches have been developed to facilitate the mechanical ventilation of the patient with ARDS. Low tidal volume ventilation uses smaller tidal volumes (6 mL/kg) to ventilate the patient, in an attempt to limit the effects of barotrauma and volutrauma. The goal is to provide the maximum tidal volume possible while maintaining end-inspiratory plateau pressure less than 30 cm H2O. To allow for adequate carbon dioxide elimination, the respiratory rate is increased to 20 to 30 breaths/min.42,43 Permissive hypercapnia uses low tidal volume ventilation in conjunction with normal respiratory rates, in an attempt to limit the effects of atelectrauma and biotrauma. Normally, to maintain normocapnia the patient’s respiratory rate would have to be increased to compensate for the small tidal volume. In ARDS though, increasing the respiratory rate can lead to worsening alveolar damage. Thus the patient’s carbon dioxide level is allowed to rise, and the patient becomes hypercapnic. As a general rule, the patient’s Paco2 should not rise faster than 10 mm Hg per hour and overall should not exceed 80 to 100 mg Hg. Because of the negative cardiopulmonary effects of severe acidosis, the arterial pH is generally maintained at 7.20 or greater. To maintain the pH, the patient is given intravenous sodium bicarbonate or the respiratory rate and/or tidal volume are increased. Permissive hypercapnia is contraindicated in patients with increased intracranial pressure, pulmonary hypertension, seizures, and heart failure.44 In pressure control ventilation (PCV) mode, each breath is delivered or augmented with a preset amount of inspiratory pressure as opposed to tidal volume, which is used in volume ventilation. Thus the actual tidal volume the patient receives varies from breath to breath. PCV is used to limit and control the amount of pressure in the lungs and decrease the incidence of volutrauma. The goal is to keep the patient’s plateau pressure (end-inspiratory static pressure) lower than 30 cm H2O. A known problem with this mode of ventilation is that as the patient’s lungs get stiffer, it becomes harder and harder to maintain an adequate tidal volume and severe hypercapnia can occur.42,43 Another alternative ventilatory mode that is used in managing the patient with ARDS is inverse ratio ventilation (IRV), either pressure controlled or volume controlled. IRV prolongs the inspiratory (I) time and shortens the expiratory (E) time, thus reversing the normal I : E ratio. The goal of IRV is to maintain a more constant mean airway pressure throughout the ventilatory cycle, which helps keep alveoli open and participating in gas exchange. It also increases FRC and decreases the work of breathing. In addition, as the breath is delivered over a longer period of time, the peak inspiratory pressure in the lungs is decreased. A major disadvantage to IRV is the development of auto–PEEP. As the expiratory phase of ventilation is shortened, air can become trapped in the lower airways, creating unintentional PEEP (or auto-PEEP), which can cause hemodynamic compromise and worsening gas exchange. Patients on IRV usually require heavy sedation with neuromuscular blockade to prevent them from fighting the ventilator.42,43 Another alternative ventilatory mode that is used in patients who remain severely hypoxemic despite the treatments previously described is high-frequency oscillatory ventilation (HFOV). The goal of this method of ventilation is similar to that of IRV in that it uses a constant airway pressure to promote alveolar recruitment while avoiding overdistention of the alveoli. HFOV uses a piston pump to deliver very low tidal volumes at very high rates or oscillations (300 to 3000 breaths/min).45 Oxygen is administered at the lowest level possible to support tissue oxygenation. Continued exposure to high levels of oxygen can lead to oxygen toxicity, which perpetuates the entire process. The goal of oxygen therapy is to maintain an arterial hemoglobin oxygen saturation of 90% or greater using the lowest level of oxygen—preferably less than 0.50.35 Because the hypoxemia that develops with ARDS is often refractory or unresponsive to oxygen therapy, it is necessary to facilitate oxygenation with PEEP. The purpose of using PEEP in the patient with ARDS is to improve oxygenation while reducing Fio2 to less toxic levels. PEEP has several positive effects on the lungs, including opening collapsed alveoli, stabilizing flooded alveoli, and increasing FRC. Thus PEEP decreases intrapulmonary shunting and increases compliance. PEEP also has several negative effects including: 1) decreasing CO as a result of decreasing venous return secondary to increased intrathoracic pressure; and 2) barotrauma, as a result of gas escaping into the surrounding spaces secondary to alveolar rupture. The amount of PEEP a patient requires is determined by evaluating both arterial hemoglobin oxygen saturation and CO. In most cases, a PEEP of 10 to 15 cm H2O is adequate. If PEEP is too high, it can result in overdistention of the alveoli, which can impede pulmonary capillary blood flow, decrease surfactant production, and worsen intrapulmonary shunting. If PEEP is too low, it allows the alveoli to collapse during expiration, which can result in more damage to alveoli.42 Extracorporeal and intracorporeal gas exchanges are last-resort techniques used in the treatment of severe ARDS when conventional therapy has failed. These methods allow the lungs to rest by facilitating the removal of carbon dioxide and providing oxygen external to the lungs by means of an “artificial lung,” or membrane/fiber oxygenator. Extracorporeal membrane oxygenation (ECMO), extracorporeal carbon dioxide removal (ECCO2R), and intravascular oxygenation (IVOX) are three techniques that employ this type of technology. ECMO is similar to cardiopulmonary bypass in that blood is removed from the body and pumped through a membrane oxygenator, where CO2 is removed and O2 is added, and then returned to the body. ECCO2R is a variation of ECMO in which the primary focus is removal of CO2. IVOX facilitates oxygenation and ventilation with the use of a fiber oxygenator that is implanted in the inferior vena cava. All of these techniques pose serious bleeding problems to the patient, and none has been shown to improve patient outcome.46,47 Adequate tissue perfusion depends on an adequate supply of oxygen being transported to the tissues. An adequate CO and hemoglobin level is critical to oxygen transport. CO depends on heart rate, preload, afterload, and contractility. A variety of fluids and medications are used to manipulate this parameter. Newer approaches to fluid management include maintaining a very low intravascular volume (pulmonary artery occlusion pressure of 5 to 8 mm Hg) with fluid restriction and diuretics, while supporting the CO with vasoactive and inotropic medications. The goal is to decrease the amount of fluid leakage into the lungs.48 Nursing management of the patient with ARDS incorporates a variety of nursing diagnoses (Box 20-6). Nursing interventions include optimizing oxygenation and ventilation, providing comfort and emotional support, and maintaining surveillance for complications. Nursing interventions to optimize oxygenation and ventilation include positioning, preventing desaturation, and promoting secretion clearance. For further discussion on these interventions, see Nursing Management of Acute Lung Failure earlier in this chapter. One additional nursing intervention that can be used to improve the oxygenation and ventilation of the patient with ARDS is prone positioning. A number of studies have shown that prone positioning the patient with ARDS results in an improvement in oxygenation. Although a number of theories propose how prone positioning improves oxygenation, the discovery that with ARDS there is greater damage to the dependent areas of the lungs probably provides the best explanation. It was originally thought that ARDS was a diffuse homogenous disease that affected all areas of the lungs equally. It is now known that the dependent lung areas are more heavily damaged than the nondependent lung areas. Turning the patient prone improves perfusion to less damaged parts of lungs and improves V/Q matching and decreases intrapulmonary shunting. Prone positioning appears to be more effective when initiated during the early phases of ARDS.49 For more information on prone positioning see Chapter 21. Collaborative management of the patient with ARDS is outlined in Box 20-7. Pneumonia is an acute inflammation of the lung parenchyma that is caused by an infectious agent that can lead to alveolar consolidation. Pneumonia can be classified as community-acquired pneumonia (CAP), hospital-acquired pneumonia (HAP), or health care-associated pneumonia (HCAP).50 Pneumonia is referred to as community-acquired when it occurs outside of the hospital or within 48 hours of admission to the hospital.51 Severe CAP requires admission to the critical care unit and accounts for about 22% of all patients with pneumonia. The mortality for this patient group is approximately 18% to 56%, with increasing age as a major risk factor.52 Pneumonia is referred to as hospital-acquired when it occurs while in the hospital for at least 48 hours.50 Ventilator-associated pneumonia (VAP) is a subgrouping of HAP that refers to development of pneumonia after the insertion of an artificial airway.50 VAP is the most common critical care unit-acquired infection.53 Pneumonia is referred to as health care-associated when it is acquired in health care environments outside of the traditional hospital setting.54 The spectra of etiologic pathogens of pneumonia vary with the type of pneumonia, as do the risk factors for the disease. Pathogens that can cause severe CAP include Streptococcus pneumoniae, Legionella species, Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus, Mycoplasma pneumoniae, respiratory viruses, Chlamydia pneumoniae, and Pseudomonas aeruginosa.51 A number of factors increase the risk for developing CAP, including alcoholism, chronic obstructive pulmonary disease (COPD), and comorbid conditions such as diabetes, malignancy, and coronary artery disease. Impaired swallowing and altered mental status also contribute to the development of CAP, because they result in an increased exposure to the various pathogens due to chronic aspiration of oropharyngeal secretions.51 Pathogens that can cause HAP include Escherichia coli, H. influenzae, methicillin-sensitive S. aureus, S. pneumoniae, P. aeruginosa, Acinetobacter baumannii, methicillin-resistant S. aureus (MRSA), Klebsiella spp., and Enterobacter spp. Two of the pathogens most frequently associated with VAP are S. aureus and P. aeruginosa. Risk factors for HAP can be categorized as host-related, treatment-related, and infection control-related (Box 20-8).55 Pathogens that can cause HCAP are similar to those causing both CAP and HAP with P. aeruginosa and MRSA being the most common in the United States.54 Risk factors for HCAP include prior hospitalization (hospitalized for 2 or more days within the last 90 days), receiving hemodialysis, receiving intravenous antibiotic therapy, chemotherapy or wound care within 30 days, residing in a long-term care facility or with family member with multidrug-resistant infection, and an immunocompromised state. These patients are at a higher risk of developing multidrug-resistant organisms.56 Development of acute pneumonia implies a defect in host defenses, a particularly virulent organism, or an overwhelming inoculation event. Bacterial invasion of the lower respiratory tract can occur by inhalation of aerosolized infectious particles, aspiration of organisms colonizing the oropharynx, migration of organisms from adjacent sites of colonization, direct inoculation of organisms into the lower airway, spread of infection to the lungs from adjacent structures, spread of infection to the lung through the blood, and reactivation of latent infection (usually in the setting of immunosuppression). The most common mechanism appears to be aspiration of oropharyngeal organisms.57 Table 20-3 lists the precipitating conditions that can facilitate the development of pneumonia. TABLE 20-3 PRECIPITATING CONDITIONS OF PNEUMONIA
Pulmonary Disorders
Acute Lung Failure
Description
Etiology
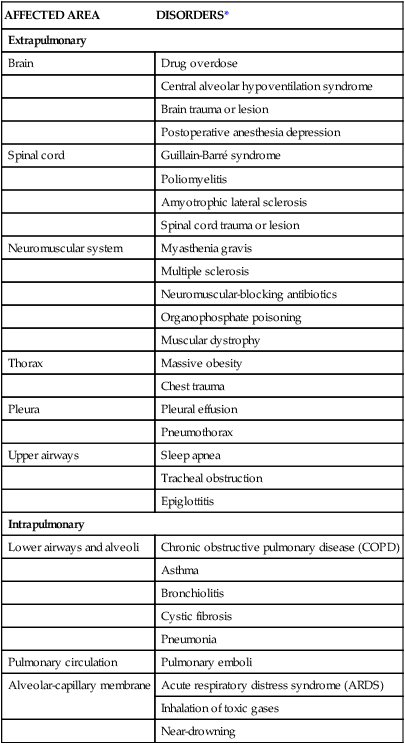
AFFECTED AREA
DISORDERS*
Extrapulmonary
Brain
Drug overdose
Central alveolar hypoventilation syndrome
Brain trauma or lesion
Postoperative anesthesia depression
Spinal cord
Guillain-Barré syndrome
Poliomyelitis
Amyotrophic lateral sclerosis
Spinal cord trauma or lesion
Neuromuscular system
Myasthenia gravis
Multiple sclerosis
Neuromuscular-blocking antibiotics
Organophosphate poisoning
Muscular dystrophy
Thorax
Massive obesity
Chest trauma
Pleura
Pleural effusion
Pneumothorax
Upper airways
Sleep apnea
Tracheal obstruction
Epiglottitis
Intrapulmonary
Lower airways and alveoli
Chronic obstructive pulmonary disease (COPD)
Asthma
Bronchiolitis
Cystic fibrosis
Pneumonia
Pulmonary circulation
Pulmonary emboli
Alveolar-capillary membrane
Acute respiratory distress syndrome (ARDS)
Inhalation of toxic gases
Near-drowning
Pathophysiology
Alveolar Hypoventilation
Ventilation/Perfusion Mismatching
Intrapulmonary Shunting
Assessment and Diagnosis
Medical Management
Oxygenation
Ventilation
Pharmacology
Acidosis
Nutrition Support
Complications
Nursing Management
Optimizing Oxygenation and Ventilation
Positioning.
Preventing Desaturation.
Promoting Secretion Clearance.
Educating the Patient and Family
Acute Respiratory Distress Syndrome
Description
Etiology
Pathophysiology
Exudative Phase
Fibroproliferative Phase
Resolution Phase
Assessment and Diagnosis
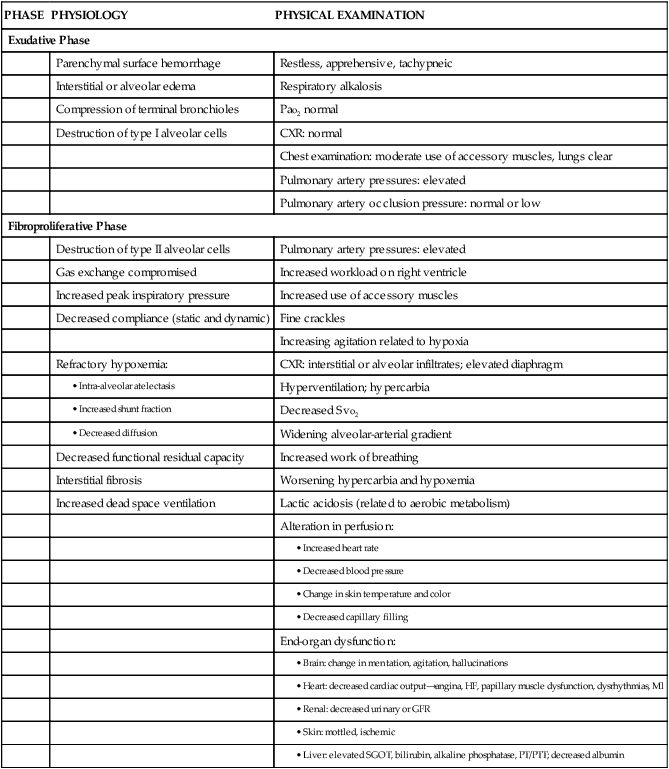
PHASE
PHYSIOLOGY
PHYSICAL EXAMINATION
Exudative Phase
Parenchymal surface hemorrhage
Restless, apprehensive, tachypneic
Interstitial or alveolar edema
Respiratory alkalosis
Compression of terminal bronchioles
Pao2 normal
Destruction of type I alveolar cells
CXR: normal
Chest examination: moderate use of accessory muscles, lungs clear
Pulmonary artery pressures: elevated
Pulmonary artery occlusion pressure: normal or low
Fibroproliferative Phase
Destruction of type II alveolar cells
Pulmonary artery pressures: elevated
Gas exchange compromised
Increased workload on right ventricle
Increased peak inspiratory pressure
Increased use of accessory muscles
Decreased compliance (static and dynamic)
Fine crackles
Increasing agitation related to hypoxia
Refractory hypoxemia:
CXR: interstitial or alveolar infiltrates; elevated diaphragm
Hyperventilation; hypercarbia
Decreased Svo2
Widening alveolar-arterial gradient
Decreased functional residual capacity
Increased work of breathing
Interstitial fibrosis
Worsening hypercarbia and hypoxemia
Increased dead space ventilation
Lactic acidosis (related to aerobic metabolism)
Alteration in perfusion:
End-organ dysfunction:
Medical Management
Ventilation
Low Tidal Volume.
Permissive Hypercapnia.
Pressure Control Ventilation.
Inverse Ratio Ventilation.
High-Frequency Oscillatory Ventilation.
Oxygen Therapy
Positive End-Expiratory Pressure.
Extracorporeal and Intracorporeal Gas Exchange.
Tissue Perfusion
Nursing Management
Optimizing Oxygenation and Ventilation
Prone Positioning.
Pneumonia
Description
Etiology
Severe Community-Acquired Pneumonia
Hospital-Acquired Pneumonia
Health Care-Associated Pneumonia
Pathophysiology
CONDITION
ETIOLOGIES
Depressed epiglottal and cough reflexes
Unconsciousness, neurologic disease, endotracheal or tracheal tubes, anesthesia, aging
Decreased cilia activity
Smoke inhalation, smoking history, oxygen toxicity, hypoventilation, intubation, viral infections, aging, COPD
Increased secretion
COPD, viral infections, bronchiectasis, general anesthesia, endotracheal intubation, smoking
Atelectasis
Trauma, foreign body obstruction, tumor, splinting, shallow ventilations, general anesthesia
Decreased lymphatic flow
Heart failure, tumor
Fluid in alveoli
Heart failure, aspiration, trauma
Abnormal phagocytosis and humoral activity
Neutropenia, immunocompetent disorders, patients receiving chemotherapy
Impaired alveolar macrophages
Hypoxemia, metabolic acidosis, cigarette smoking history, hypoxia, alcohol use, viral infections, aging
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pulmonary Disorders
Get Clinical Tree app for offline access