Principles of intrapartum skills
Third-stage issues
Learning outcomes
Having read this chapter, the reader should be able to:
• describe the physiology of the third stage of labour
• discuss the effects of delayed cord clamping and umbilical cord milking on the baby
• discuss the different methods of managing the third stage
• discuss how blood loss is estimated
• describe how the genital tract is examined following delivery.
For the majority of women the third stage of labour occurs with no adverse outcomes but it has the potential to be a hazardous time for the woman, as primary postpartum haemorrhage (PPH) remains a direct cause of maternal mortality. While in the UK the risk of death from haemorrhage is very small (11 women died from PPH during 2010–2012) (Knight et al 2014), worldwide, particularly in developing countries, the death rate can be much higher, accounting for 30% of maternal deaths in Africa and Asia (Fawole et al 2012). The midwife has a responsibility to ensure the placenta and membranes are delivered safely and competently following the delivery of the baby. This chapter focuses on the principles of the management of the third stage of labour, discussing both expectant and active management, examination of the genital tract following delivery of the placenta and estimation of blood loss; relevant anatomy and physiology are included.
Physiology of the third stage of labour
The third stage is from the birth of the baby to the complete expulsion of the placenta and membranes, involving the separation, descent, and expulsion of the placenta and membranes; the control of haemorrhage from the placental site; and examination of the genital tract following delivery. Perineal repair is occasionally undertaken while awaiting the birth of the placenta.
The physiology of placental separation has been revised in recent years following ultrasound visualization of placental separation (Herman 2000, Herman et al 2002, Krapp et al 2000) with three phases identified: latent, contraction/detachment, and expulsion. The intrauterine volume reduces drastically (from 4 L prelabour to 0.5 L) as the uterus becomes smaller following the delivery of the baby. Pressure within the uterus increases from 100 mmHg in the second stage to 140 mmHg in the third stage.
Phase 1: latent phase
The myometrium continues to contract and retract as with the first and second stages of labour resulting in extensive thickening of most of the myometrium; the area of myometrium beneath the placental site does not thicken to the same degree. This takes 101 ± 87 seconds.
Phase 2: contraction/detachment phase
The myometrium under the lower pole of the placenta begins to contract with a reduction in the surface area. Consequently, the shearing forces cause the placenta to separate from the spongy layer of the decidua. With the onset of placental detachment, the wave of separation passes upwards and the remaining placenta detaches, with the uppermost part of the placenta detaching last, leaving the maternal sinuses within the decidua exposed. The oblique muscle fibres surrounding the blood vessels contract to seal the torn ends of the maternal vessels to prevent haemorrhage. This phase lasts 56 ± 45 seconds.
Phase 3: expulsion phase
As the placenta descends into the lower uterine segment, the membranes (which had begun to detach from the uterine wall as the internal cervical os dilated) peel away from the walls of the uterus. With further contractions the placenta descends into the vagina, assisted by gravity, with the membranes following. This takes 77 ± 63 seconds.
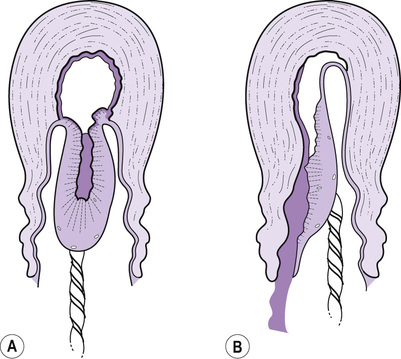
Maternal effort will birth the placenta and membranes with the fetal surface appearing at the vulva, with the membranes behind containing any blood loss within them; this is often referred to as the ‘Schultze’ method of expulsion (Fig. 32.1A). Sometimes the lower edge of the placenta will descend first with the maternal surface appearing at the vulva and sliding out lengthways with the membranes (Fig. 32.1B). This is a slower process, with increased blood loss as the mechanisms to control haemorrhage are less effective when the placenta is still partially attached. This has been referred to as the ‘Matthews Duncan’ method of expulsion.
Radiological studies have demonstrated that the placenta usually separates within 3 minutes of the birth of the baby (Brandt 1933). Herman (2000) suggests the duration of the third stage varies according to the length of the latent phase; however, the time taken for the descent and birth of the placenta and membranes can also vary individually, influenced by factors such as posture and whether the third stage is managed actively or expectantly. Harris (2011) suggests the placenta begins to separate with the birth of the baby and is completed within one to two contractions.
Signs of separation and descent
These are not absolute and may occur for other reasons:
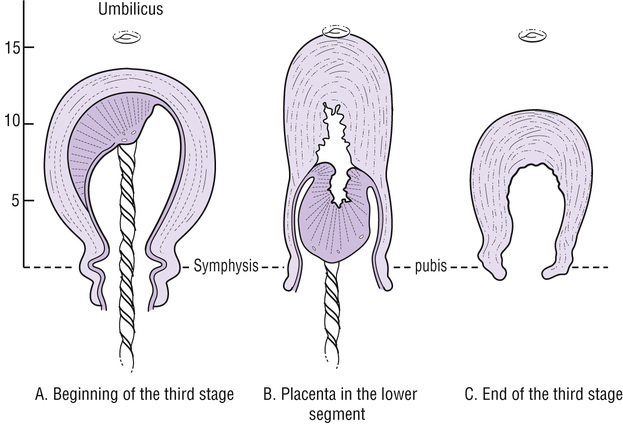
• A change in the shape and position of the uterus: the uterus becomes globular, hard, high, mobile and ballottable. Before placental separation and descent into the lower uterine segment, the fundus is broad and palpable, usually below the umbilicus. With separation and descent, the fundus narrows and fundal height increases, usually above the umbilicus (Fig. 32.2). This can be assessed by gently palpating the fundus (it may provoke irregular contractions which can interfere with placental separation and cause a partially separated placenta, resulting in excessive bleeding). The placenta may also appear as a bulge just above the symphysis pubis.
• The woman feels pressure or an urge to push as the placenta enters the vagina.
Control of haemorrhage
The placental circulation is approximately 500–800 mL/min at term; thus bleeding from the placental site can be profuse and rapid and it is vital that haemorrhage is controlled. This is achieved in three ways:
Cord clamping
With the introduction of active management, the practice of immediate cord clamping following birth (within 30 seconds) became widespread. However, placental separation is reliant on the ability of the uterus to continue to contract and retract. Clamping the cord can set up a counter-resistance in the placenta preventing the transfer of blood to the baby. This can prevent the placenta from reducing in size with the potential to inhibit contraction and retraction of the uterus resulting in a slower separation process. This has two effects:
When the cord is not clamped immediately, the process of placental separation is unaffected and the baby receives the extra blood contained within the placenta. Scheans (2013) suggests that blood flow via the umbilical arteries from baby to placenta stops after 20–25 seconds following birth, whereas the blood flow from the placenta to the baby via the umbilical vein continues for up to 3 minutes. McAdams (2014) refers to the importance for the baby of receiving this additional iron-rich blood, suggesting the first minute before cord clamping is the ‘iron minute’.
When it is time to clamp the cord, the clamp should be applied 3–4 cm from the abdominal wall. If the baby is preterm the cord should be longer, as catheterization of the umbilical vessels may be required; this is more successful with a longer cord. A clamp is usually applied to the maternal end of the cord and the section of cord between the two clamps cut. This may be something that the woman’s partner or family member will want to do and the midwife should direct them where to cut.
Delayed cord clamping (DCC)
Short-term benefits
DCC has many short-term benefits. The most obvious benefit is the increased blood volume (ACOG 2012, Meyer & Mildenhall 2012, Scheans 2013). Mercer et al (2010) suggest the very low birthweight baby receives 10–15 mL/kg of extra blood with even a brief delay in cord clamping while Raju (2013) advises the amount increases with time, suggesting 16 mL/kg by 60 seconds and 23 mL/kg by 180 seconds for the term baby. Airey et al (2008) propose DCC increases placental transfusion to the baby by 20%, providing an extra 20–40 mg/kg of iron for the term baby whereas Blouin et al (2013) advise the extra 15–40 mL/kg of blood received increases blood volume by 30–50% and provides an additional 30–75 mg of iron with 3 minutes of DCC. McDonald et al (2013) suggest blood volume increases by 30% and the baby receives 60% more red blood cells. This can significantly increase the haemoglobin for both the preterm (Aziz et al 2012, Ghavam et al 2014) and the term (Raju 2013) baby and increases circulating ferritin levels (Raju 2013) and iron stores at 2–4 months (Raju 2013), decreasing the risk of iron-deficiency anaemia between 4–6 months (Scheans 2013) and the need for blood transfusions (ACOG 2012, Ghavam et al 2014, Rabe et al 2012, Raju 2013, Scheans 2013). The extra blood volume also assists with facilitating the pulmonary adaptation required at and following birth and Baenziger et al (2007) suggest this is why there is less need for medical interventions such as mechanical ventilation in the preterm baby following DCC.
The increased blood volume improves blood flow in the superior vena cava and may decrease vascular resistance, improving the preterm baby’s ability to autoregulate cerebral blood flow in early life (Meyer & Mildenhall 2012). Blood pressure is increased for the preterm baby (within normal limits) (Ghavam et al 2014, Rabe et al 2012, Raju 2013) possibly reducing the risk (up to 50%) of intraventricular haemorrhage for the preterm baby who has DCC (ACOG 2012, Ghavam et al 2014, Rabe et al 2012, Raju 2013, Scheans 2013). There is improved cerebral perfusion and oxygenation (Baenziger et al 2007, Raju 2013) which potentially improves perfusion generally, reducing the risk of organ injury occurring from decreased perfusion. Overall there is also a decreased incidence of intracranial haemorrhage (ACOG 2012, Raju 2013, Scheans 2013).
Tolosa et al (2010) refer to the transfusion of placental blood as ‘nature’s first stem cell transplant’. The stem cells within the blood have anti-inflammatory, neurotrophic, and neuroprotective effects (Raju 2013, Tarnow-Mordi et al 2014). Ghavam et al (2014), Rabe et al (2012) and Scheans (2013) found DCC helps reduce the incidence of late-onset sepsis and necrotizing enterocolitis for preterm babies.
Other benefits include increased duration of early breastfeeding for preterm babies <32 weeks (Mercer et al 2006), significantly less hypothermia (Aziz et al 2012), and increased urinary output in the first 48 hours (Raju 2013).
From the maternal perspective, McDonald et al (2013) found no significant difference in the incidence of severe PPH or haemorrhage of 500 mL and no significant difference in mean blood loss at delivery or postnatal maternal haemoglobin levels when DCC was undertaken for term babies.
Long-term benefits
There is little evidence currently for or against longer-term benefits. Blouin et al (2013) reviewed the effect of DCC on babies whose mothers were anaemic compared to non-anaemic mothers. They found at 8 months there was significantly less iron deficiency in babies who had DCC and whose mothers were anaemic. Mercer et al (2010) suggest that DCC seems to be a protective factor against motor disability at 7 months corrected age for very low birthweight infants (they studied babies at 24–31+6 weeks’ gestation); however, Ghavam et al (2014) found no difference in disability between babies who had early or late delayed cord clamping or cord milking. Andersson et al (2013a) agree, they found no difference at 4 months on neurodevelopment of symptoms of infection.
Risks of delayed cord clamping
Tarnow-Mordi et al (2014) are concerned about an increase in the incidence and severity of jaundice, a view supported by Raju (2013) who suggests there is an increased need for phototherapy. However, Scheans (2013) and Cernadas et al (2006) found asymptomatic polycythaemia, (haematocrit >65%) was more common in babies who had DCC but this was not associated with a significant difference in serum bilirubin levels or the need for phototherapy in the first 1–3 days.
In certain situations DCC may not be advisable, e.g. where there are maternal antibodies circulating and are destroying fetal cells, as the baby receives not only extra red blood cells during the placental transfusion of blood but also everything else in the blood. These cases are less common in the developed world and are considered on an individual basis.
Duration of DCC
If the third stage is expectant, clamping and cutting the cord would not occur until after the cord has stopped pulsating. For the baby having a lotus birth (see Chapter 33), there would be no clamping or cutting of the cord. But if the third stage is to be actively managed, how long should the cord pulsate and transfer blood before it is clamped and cut?
The consensus appears to be that DCC should take somewhere between ≥30 seconds and up to or longer than 3 minutes (ACOG 2012, McAdams 2014, Raju 2013, Scheans 2013, WHO 2014) for a term baby. The longer the cord pulsates, the greater the placental transfusion of blood to the baby. ACOG (2012) suggest the ideal duration for the preterm baby has not been established and the need for resuscitation overrides DCC; Rabe et al (2012) and WHO (2014) suggest it should be for 30–120 seconds.
Position of the baby
Early studies recommended positioning the baby 40 cm below the introitus for 30 seconds for maximal transfer of blood (Palethorpe et al 2010). More recent studies of DCC position the baby at the level of the introitus for a vaginal birth (McAdams 2014, Meyer & Mildenhall 2012) or below the introitus (Andersson et al 2013b, Mercer et al 2010) and above the level of the uterus, usually on the maternal legs, following a caesarean birth (McAdams 2014, Meyer & Mildenhall 2012). Blouin et al (2013), however, positioned the baby on the mother’s abdomen, which is more realistic of what happens following the vaginal birth of the baby, a position supported by Cook (2007). Palethorpe et al (2010) found no reliable research to demonstrate whether the position of the baby during DCC makes a difference to the health of the baby or the mother. It would make sense to continue placing the baby skin-to-skin with his mother during DCC, with its known benefits (Moore et al 2012) until good evidence suggests otherwise.
Milking the cord
Tarnow-Mordi et al (2014) suggest that milking the umbilical cord disrupts the fetoplacental circulation and the transition of the cardiopulmonary and cerebral circulation. However, in situations where there is no time for DCC, e.g. resuscitation, milking the cord may offer some advantages (Raju 2013). But how much of the cord should be milked, how quickly, and how many times? And does it vary according to gestational age? Much of the research around milking the cord involves preterm babies who are likely to experience early cord clamping due to resuscitation needs but who would benefit from delayed cord clamping – 24–28 weeks (March et al 2013), 32 weeks (Backes et al 2010).
March et al (2013) held babies at or below the level of the placenta following a vaginal birth and at the same level following an operative birth; 20 cm of cord was milked three times. Patel et al (2014) held babies 10 cm below the placenta, pinching the cord as close to the placenta as possible and milking it towards the baby for 2–3 seconds, then released the cord for 2–3 seconds and repeated twice more but for <30 seconds in total. Upadhyay et al (2013) undertook cord milking on babies who were placed on a resuscitaire by leaving the cord 25 cm long. The cord was then milked three times at 10 cm/sec after which it was clamped. Takami et al (2012) used a 20 cm segment of cord two to three times at 20 cm/sec.
Advantages of cord milking include reduced rates of mortality (Backes et al 2010), intraventricular haemorrhage (IVH) (Backes et al 2010, March et al 2013), transfusion (IVH) (Backes et al 2010, March et al 2013), higher haemoglobin (Hb) and serum ferritin at 6 weeks (Upadhyay et al 2013), and relatively higher blood pressure (within the normal range) during the first 48 hours of life (Upadhyay et al 2013). Higher initial Hb, mean arterial blood pressure, and urine output levels within the first 24 hours decreased the need for volume expanders (Patel et al 2014, Takami et al 2012). Takami et al (2012) conclude that cord milking improves cerebral perfusion. Additionally there is no increased risk of hyperbilirubinaemia and phototherapy (March et al 2013).
Backes et al (2010) advise caution though, as the effects of milking on the fragile germinal matrix vessels in the preterm baby are unknown and suggest this warrants further consideration in future studies.
Management of the third stage
There are considerable variations between and within countries in policies for the management of the third stage of labour and the pharmacological agents used (Winter et al 2007). Midwives should be competent with both expectant (physiological, passive) and active management (RCM 2012).
Expectant management of the third stage of labour (EMTSL)
Women who have experienced a physiological labour and birth can be offered a physiological third stage for birthing their placenta (NZCOM 2013, RCM 2012); the physiological process of placental separation and delivery are dependent on a finely tuned balance of hormonal, physiological, psychological and neurological interactions, which if any of these have been disturbed, can compromise safety at this time (Fry 2007). With EMTSL the placenta is delivered by maternal effort assisted by gravity and the baby suckling at the breast. There should be no intervention – no drugs, no cord clamping (unless it has stopped pulsating), no palpation of the abdomen. Signs of separation and descent are usually seen.
A calm, warm, relaxed environment with skin-to-skin contact with the baby (NZCOM 2013, RCM 2012) should be maintained so that fear, anxiety, and tension are dissipated. If not, adrenaline levels increase which can inhibit oxytocin release and interfere with the physiological process of uterine contraction and retraction, and thus placental separation and descent (Blackburn 2008, Buckley 2004, Page 2007). Indeed, Kanikasamy (2007) advises oxytocin release is enhanced when the midwife is calm and confident and able to instill confidence. Blackburn (2008) agrees, suggesting the woman should be undisturbed to lower catecholamine levels and encourage oxytocin and prolactin release.
EMTSL can be undertaken with preterm labour; however, it is acknowledged that this may not be possible if the baby requires active resuscitation as the cord may require early clamping. However, the maternal end of the cord can be left unclamped (place the cord in a sterile receiver) to facilitate a limited amount of blood to drain from the placenta, helping to reduce its overall size. According to Lucas (2006), this helps minimize retroplacental bleeding and decreases the risk of partial separation and isoimmunization. The other principles of EMTSL are the same, but this is not as effective and may inhibit the physiological process. In the presence of haemorrhage, a uterotonic drug is required and a move to actively managing the third stage. The blood drained from the placenta should not be included in the total estimate of blood loss following delivery, being placental and not maternal blood.
Blood loss is higher with EMTSL (Begley et al 2015); however, if the woman is not compromised and the loss not severe, it may be a physiological loss which the body can cope with. Wickham (1999) suggests blood loss in the early postnatal period will be less when the third stage is managed expectantly compared with active management. However, it is important to discuss this with the woman, ideally during the antenatal period or early labour, as the midwife should change to active management if the woman begins to haemorrhage. As such, the woman should be aware of the risk of haemorrhage and consent to the change from expectant to active management in the presence of haemorrhage.
The duration of the third stage is often longer with EMTSL. Dixon et al (2009) suggest that in the absence of bleeding, it can last more than an hour without an increased risk of PPH. While the woman’s condition remains stable, with no excessive bleeding, there is no cause for concern; breastfeeding can be initiated, with the added benefit of increased oxytocin release to promote uterine contraction. The RCM (2012) recommend the woman should be encouraged to adopt an upright position to shorten the duration of the third stage. NICE (2014) suggest the placenta should be delivered within 1 hour and if this does not happen, management should change to active. At this point the midwife should assess whether the placenta has separated by positioning the woman on her back and, using only fingertips, gently pressing her abdominal wall just above the pubic bone and observing the cord. If the cord does not move, Odent (1998) advises the placenta has separated.
Principles of expectant management
• The principles of standard precautions (Chapter 8) and an Aseptic Non Touch Technique (ANTT) (Chapter 10) are followed to reduce the risk of infection.
• Note the time of delivery of the baby.
• Keep the baby covered on the woman’s abdomen, skin-to-skin.
• Maintain a safe, warm, private environment, taking steps to reduce any anxiety in the woman.
• Ensure the woman’s bladder is empty.
• Encourage the woman to adopt an upright position.
• Observe the general condition of the woman throughout, particularly blood loss per vaginam, colour, respirations (NICE 2014).
• Do not touch the cord, allowing it to stop pulsating naturally.
• Encourage and assist the woman to breastfeed when the baby is ready.
• Do not palpate the uterus unless blood loss becomes excessive.
• Note the time the placenta and membranes are expelled.
• The cord can be clamped and cut when it has stopped pulsating, unless the woman has requested a lotus birth (Chapter 33).
• Examine the placenta (Chapter 33) and record total blood loss.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree