32. Principles of intrapartum skills
third-stage issues
CHAPTER CONTENTS
Estimation of blood loss231
Role and responsibilities of the midwife233
Summary233
Self-assessment exercises233
References233
LEARNING OUTCOMES
Having read this chapter the reader should be able to:
• describe the physiology of the third stage of labour
• discuss the effects of early and delayed cord clamping on the baby
• discuss the different methods of managing the third stage
• discuss how blood loss is estimated
• describe how the genital tract is examined following delivery.
The third stage of labour can be a dangerous stage for the woman as primary postpartum haemorrhage (PPH) remains a cause of maternal mortality and mismanagement of the third stage increases the risk. In the UK, the risk of death from haemorrhage is very small (nine women died from PPH during 2003–2005) (Lewis 2007). However, worldwide, particularly in developing countries, the death rate can be much higher, accounting for 11% of maternal deaths (Lewis 2007). Following the delivery of the baby, the midwife has a responsibility to ensure the placenta and membranes are delivered safely and competently. This chapter focuses on the principles of the management of the third stage of labour, discussing both physiological and active management, examination of the genital tract following delivery of the placenta and estimation of blood loss; relevant anatomy and physiology are included.
Physiology of the third stage of labour
Placental separation has previously been thought to result from the placenta being squeezed following the reduction in the size of the uterus at the beginning of the third stage. This was thought to result in an increased pressure within the blood vessels of the spongy layer of the decidua, causing them to rupture. The escaping blood between the placental surface and the thin septa of the spongy layer resulted in the placenta shearing from the decidua. However, following ultrasound visualisation of placental separation, a different understanding of the physiology has emerged (Herman, 2000, Herman et al., 2002 and Krapp et al., 2000) with three phases identified: latent, contraction/detachment and expulsion.
With the delivery of the baby, the intrauterine volume reduces drastically (from 4 L before labour to 0.5 L) as the uterus becomes smaller. Intrauterine pressure increases from 100 mmHg in the second stage to 140 mmHg in the third stage.
Phase 1: latent phase
Contraction and retraction of the myometrium continues as with the first and second stages of labour causing extensive thickening of most of the myometrium. However, the area of myometrium beneath the placental site is unable to thicken to the same extent.
Phase 2: contraction/detachment phase
With further contraction and retraction, the myometrium under the lower pole of the placenta begins to contract with a reduction in the surface area. Consequently, the shearing forces cause the placenta to tear away from the spongy layer of the decidua. With the onset of placental detachment, the wave of separation passes upwards and the remaining placenta detaches, with the uppermost part of the placenta detaching last. At this point, the maternal sinuses within the decidua are exposed. The oblique muscle fibres surrounding the blood vessels contract, sealing off the torn ends of the maternal vessels, helping to prevent haemorrhage.
Phase 3: expulsion phase
The placenta descends into the lower uterine segment, causing the membranes (which had begun to detach from the uterine wall as the internal cervical os dilated) to peel away from the walls of the uterus. As the uterus contracts, the placenta descends into the vagina, assisted by gravity, with the membranes following.
The placenta and membranes are then expelled by maternal effort. The fetal surface appears first at the vulva, with the membranes behind, and any blood loss is contained within this – the ‘Schultze’ method of expulsion (Fig. 32.1A). Sometimes the lower edge of the placenta descends first, so that the maternal surface appears at the vulva, sliding out lengthways with membranes (Fig. 32.1B). This is a slower process, with increased blood loss (the mechanisms to control haemorrhage are less effective when the placenta is still partially attached) – also known as the ‘Matthews Duncan’ method of expulsion.
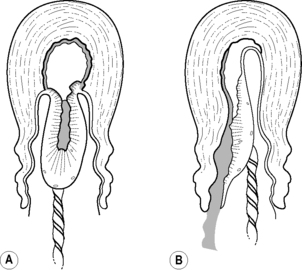 |
| Figure 32.1 • Methods of placental expulsion. A Schultze. B Matthews Duncan |
Brandt (1933) demonstrated by radiographic studies that the placenta usually separates within 3 minutes of the birth of the baby. Herman (2000) suggests that the duration of the third stage is dependent on the length of the latent phase; however, the time taken for the descent and expulsion of the placenta and membranes can also vary individually, influenced by factors such as posture and whether the third stage is managed actively or expectantly.
Signs of separation and descent
These are not absolute and may occur for other reasons:
• Bleeding: 30–60 mL of blood may trickle from the vagina: this may also occur with a partially separated placenta, although bleeding is often heavier, or from a laceration.
• Lengthening of the cord: this occurs as the placenta descends, but may also occur if the cord is coiled and then straightens out.
• Uterus becomes globular, hard, high, mobile and ballottable: this is assessed by palpating the fundus but should be undertaken with caution as it may cause irregular contractions, resulting in a partially separated placenta and membranes, and excessive bleeding. The fundus is palpable below the umbilicus, and is broad, until placental separation and descent into the lower uterine segment. The fundal height increases, usually above the umbilicus, with the fundus narrowing (Fig. 32.2).
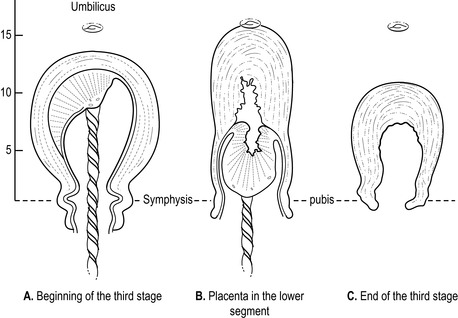 |
| Figure 32.2 • Position of the uterus before and after placental separation |
Control of haemorrhage
Bleeding from the placental site can be profuse and rapid, as the placental circulation is approximately 500–800 mL/minute at term. It is imperative that haemorrhage is controlled. The body attempts to do this in three ways:
1. The middle oblique fibres of the uterus contract and retract, constricting the blood vessels running through them. This causes the vessels to kink, slowing down and stopping the blood flow, allowing for clot formation at the placental site.
2. The walls of the uterus become in apposition to each other, exerting pressure on the placental site.
3. The blood clotting mechanism begins to work at the placental site, within the sinuses and torn vessels. The damaged tissue releases thrombokinase, converting prothrombin to thrombin. This combines with fibrinogen to form fibrin, which then combines with platelets to form a clot. Vitamin K, calcium and the other clotting factors are required for this process to happen efficiently.
Clamping of the umbilical cord
The custom of cutting the cord was introduced in the seventeenth century. Its introduction coincided with the practice of giving birth in bed, which resulted in the bedding becoming blood-soaked; the practice of clamping the cord became widespread to reduce this. With active management, the cord is clamped immediately after delivery, usually within 30 seconds; with expectant management, it is not cut until it has stopped pulsating (McDonald & Middleton (2008) suggest this is often within 2 minutes). There is much debate currently regarding the timing of cord clamping for active management as evidence is emerging that delayed clamping (30 seconds to 5 minutes after delivery) has a beneficial effect on the health and wellbeing of the baby (see below). An increasing number of women are opting for a ‘lotus birth’, which leaves the baby attached to the placenta and cord until the cord shrivels and detaches naturally from the umbilicus (Cook 2007).
Placental separation relies on the ability of the uterus to contract and retract. If the cord is clamped, a counter-resistance is set up in the placenta, preventing the transfer of blood to the baby. The size of the placenta does not reduce as much and this can inhibit contraction and retraction, resulting in a slower separation process. The effect of this is twofold:
1. The delay in complete separation means there is delay in sealing off the torn maternal vessels, resulting in a retroplacental clot and increasing the risk of haemorrhage.
2. The cervix may retract before the placenta is expelled, resulting in a retained placenta, which often necessitates a manual removal of the placenta and membranes under an epidural, spinal or general anaesthetic.
Clamping the cord and Rhesus isoimmunisation
When the cord is clamped, more fetal blood remains in the placenta, increasing the pressure within the placenta. As the uterus contracts, the pressure increases further and the surface placental vessels rupture. Fetal blood cells are released into the uterine cavity and may pass into the maternal circulation. Thus active management can increase the risk of fetomaternal transfusion (Enkin et al 2000). If the baby is Rhesus positive and the woman is Rhesus negative, she will produce antibodies against the Rhesus-positive blood cells. Rhesus isoimmunisation can affect future pregnancies, as the antibodies are small enough to pass through to the placenta and haemolyse fetal cells if the fetus is Rhesus positive. All Rhesus-negative women should receive anti-D immunoglobulin at birth if the baby is Rhesus positive to reduce the risk of isoimmunisation occurring. If antibodies are already present and known to be haemolysing fetal red blood cells, the cord should be clamped immediately at birth to reduce the number of antibodies transferred to the baby at delivery. This may help to reduce the severity of anaemia and jaundice occurring from isoimmunisation.
Clamping the cord: the effect on the baby
Airey et al (2008) suggest delayed cord clamping increases placental transfusion to the baby by 20%, the equivalent of an extra 20–30 mL/kg blood and 20–40 mg/kg of iron for the term baby. This extra blood may be required for the newly established pulmonary circulation. Early cord clamping reduces the amount of blood transferred to the baby, with resulting hypovolaemia, which may be a factor in the development and severity of respiratory distress syndrome and can compromise the baby who is born with low haemoglobin.
There are many benefits associated with delayed cord clamping. For the term baby there is increased tissue oxygenation within the first week of life, increased haematocrit, haemoglobin and blood volume, reduced incidence of anaemia at 2 months and beyond which is thought to improve the baby’s resistance to infection and aid neurodevelopment in infancy – for some babies this can be the difference between life and death in infancy (Airey et al., 2008, Cook, 2007 and Weckert and Hancock, 2008). With the preterm baby, delayed cord clamping is associated with a reduced need for blood transfusion and ventilation, decreased incidence of hypotension, anaemia, intraventricular haemorrhage, improved cerebral oxygenation, increased blood volume (20–60% increase in red blood cells), haemoglobin levels (at 1 hour, 10 weeks and 6 months) and ferritin levels (Airey et al., 2008, Baenziger et al., 2007, Chapparo et al., 2006, Cook, 2007, McDonald and Middleton, 2008, Rabe et al., 2004 and Ultee et al., 2006). The increase in early protein and iron levels with a 50% reduction in the number of blood transfusion needs for very low birthweight babies was found even with a delay in clamping of 30 seconds (Rabe et al 2009).
To achieve optimum placental transfusion, Cook (2007) suggests the baby should be delivered onto the woman’s abdomen. Airey et al (2009) suggest the earlier studies looking at placental-baby blood flow which suggested the baby should be placed 40 cm below the introitus for 30 seconds were undertaken when ergometrine was the drug used to manage the third stage. However, as the blood flow in the umbilical vein, which transfers placental blood to the baby increases as the uterus contracts, Airey et al (2009) suggest that keeping the baby level with the placental bed, or within 10 cm above or below, has little effect on the size and speed of placental transfusion. They are currently undertaking a review of the studies comparing different positions of the baby relative to placental position prior to cord clamping – the reader is advised to look for their findings on the Cochrane database when they are published.
There is a risk of overtransfusing red blood cells predisposing the baby to jaundice particularly when an oxytocic drug is given; however Airey et al (2008) question whether there is an increased requirement to treat the jaundice. Cernadas et al (2006) found increased levels of polycythaemia although no babies were symptomatic or required treatment; additionally these differences did not persist beyond 24–48 hours and there were no significant differences in bilirubin levels when the cord was clamped immediately or at 1 or 3 minutes. However McDonald & Middleton (2008) found an increase in jaundice requiring treatment with phototherapy.
Cord clamping and maternal outcome
Clamping the cord immediately following the birth of the baby or delaying clamping up to 3 minutes makes no significant difference to PPH rates (McDonald & Middleton 2008). Cernadas et al (2006) also found that delayed cord clamping made no difference to maternal outcomes. Draining the placenta is associated with a shorter third stage and a decreased risk of retained placenta (Soltani et al 2005), although further research into these effects is needed.
Management of the third stage
This is either expectant (physiological, passive) or active.
Expectant management
This is delivery of the placenta without intervention: no oxytocic drug, no cord clamping unless it has stopped pulsating, no controlled traction, no palpation of the abdomen. The placenta is delivered by maternal effort, assisted by gravity and the baby suckling at the breast. Signs of separation and descent are seen.
Women should only be considered for expectant management if they have had a physiological first and second stage as Fry (2007) advises that the physiological process of placental separation and delivery are dependent on a finely tuned balance of hormonal, neurological, physiological and psychological interactions. If any of these have been disturbed, Fry (2007) suggests the safety of the third stage is compromised; this includes the use of syntocinon which desensitizes oxytocin receptors (Gyte 2006). Equally the environment needs to be private and warm, with fear, anxiety and tension dissipated otherwise adrenaline levels increase which can inhibit oxytocin release and interfere with the physiological process of uterine contraction and retraction, and thus placental separation and descent (Blackburn, 2008 and Buckley, 2004). Kanikasamy (2007) suggests that a calm confident midwife who is able to instil confidence and practise non-invasive care will promote the release of oxytocin. Blackburn (2008) agrees, suggesting that the woman should be undisturbed, as this causes catecholamine levels to reduce whilst high levels of oxytocin and prolactin are released. Uninterrupted skin-to-skin contact and suckling should also be encouraged as this promotes a surge in oxytocin release (Blackburn, 2008 and Page, 2007). Expectant management can be undertaken with preterm labour; however, it is acknowledged that this may not be possible if the baby requires active resuscitation.
Expectant management is associated with a higher blood loss (Prendiville et al 2000), partly because blood loss measurement is more accurate. Provided this is not excessive and the woman is not compromised, it may be a physiological loss with which the body can cope. Wickham (1999) suggests that the blood loss in the early postnatal period is less when the third stage is managed expectantly compared with active management. However, it is important to discuss this with the woman, ideally during the antenatal period or early labour, as active management should ensue if the woman begins to haemorrhage. The woman should be aware of the risks and consent to change from expectant to active management in the presence of haemorrhage.
Expectant management can take longer to complete than one that is actively managed – possibly up to 1 hour. Provided the woman’s condition remains stable, with no excessive bleeding, there is no cause for concern. This may be a time when breastfeeding is initiated, with the added benefit of increased oxytocin release to promote uterine contraction. The National Institute for Health and Clinical Excellence (NICE 2007




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
