Chapter 7 Principles of hemodialysis
Historical background
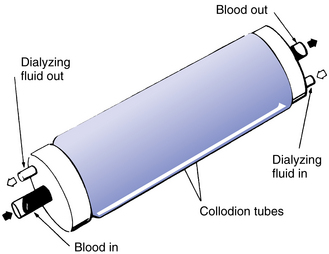
Thomas Graham, a London chemist, reported the principles of the semipermeable membrane in 1861 and gave the process of selective diffusion the name dialysis. Then, in 1913, Abel, Rowntree, and Turner devised an apparatus for the dialysis of blood, using a number of collodion tubes, through which flowed blood while a saline solution bathed the outsides of the tubes (Fig. 7-1). This device was used successfully to treat animals with uremia. Later, Kolff and Berk developed the first clinically successful artificial kidney, after the development of heparin for anticoagulation and the ready availability of cellulose in the form of cellophane tubing. They employed a rotating drum of wood slats around which a spiral of cellophane tubing was wrapped. The lower portion of the drum was immersed in a bath of dialysis fluid, while the blood was propelled along the tubing by rotating the drum. In 1948 Skeggs and Leonards developed a parallel plate dialyzer; the first disposable dialyzer was the Travenol twin-coil unit, marketed in 1956. About 1965 Gambro began production of disposable parallel plate devices, while at the same time hollow-fiber artificial kidneys were developed in the U.S.
Solute transfer
What waste products are removed by dialysis?
A large number of substances accumulate in uremia (see Chapter 4). The molecular size of many of these substances is less than 500 daltons (Da). A dalton is a unit of mass and is sometimes called atomic mass unit (amu). Daltons diffuse readily across cellulosic membranes. Particles in the range of 500 to 2000 Da, sometimes called middle molecules, diffuse poorly across such membranes. Polypeptides in this size range have been suspected of causing some uremic symptoms, although this has never been proven. Molecules larger than 3000 Da are not generally regarded as toxic, with the exception of β2-microglobulin (11,800 Da) and its relation to amyloidosis, bone disease, and anemia. See Table 7-1 for the molecular weight of some common substances.
Table 7-1 Molecular Weights of Some Common Substances
| Substance | Molecular weight (Da) |
|---|---|
| Acetylsalicylic acid (aspirin) | 180 |
| Albumin | 68,000 |
| β2-microglobulin | 11,600 |
| Cholesterol | 386 |
| Creatinine | 113 |
| Dextrose | 198 |
| Glucose | 180 |
| Hemoglobin | 68,800 |
| Urea | 60 |
| Vancomycin | 1486 |
Adapted from Daugirdas, JT: Handbook of dialysis, Philadelphia, 2007, Lippincott.
What factors affect the diffusion or removal of toxins in dialysis?
• Dialysate temperature: The higher the temperature, the greater the solute removal.
• Dialysate flow rate: The greater the dialysate flow rate, the greater the removal of solutes.
• Blood flow rate: The greater the blood flow rate, the greater the removal of solutes.
• Molecular weight of solutes: The smaller the molecular weight, the greater the removal of solutes.
• Concentration gradient: The greater the concentration gradient, the greater the amount of diffusion.
• Membrane permeability: The more permeable the membrane, the greater the removal of solutes.
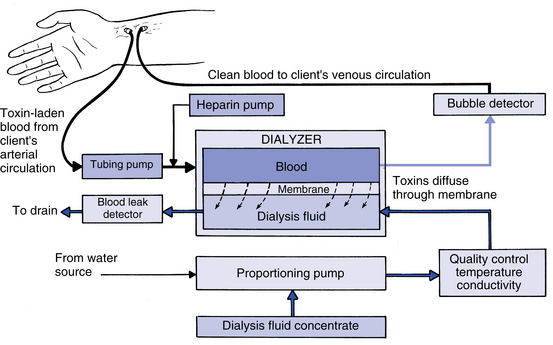
How does the semipermeable membrane function in hemodialysis?
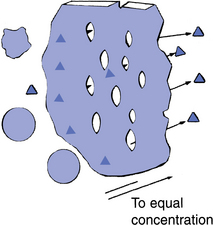
The patient’s blood is passed through a compartment formed by the semipermeable membrane. Dialyzing fluid surrounds this compartment. Red blood cells, white blood cells, platelets, and most plasma proteins are too large to pass through the pores of the membrane. Water and small particles, such as electrolytes, cross by diffusion (Fig. 7-2), as do urea (60 Da), creatinine (113 Da), and glucose (184 Da).
What is diffusion?
Diffusion, or conductive transport, may be defined as the movement of solutes from an area of greater concentration of solutes to an area of lesser concentration of solutes. Molecules in solution are in constant motion and seek to spread uniformly throughout the solution. The rate of spread depends on the concentration, size, and electric charge of the particles. Diffusion of particles across a semipermeable membrane is the basis of dialysis. Diffusion will occur until equilibrium is reached (Fig. 7-3).
Are membranes permeable to middle-size and large molecules?
Several synthetic materials are used for high-flux dialysis. These include polyacrylonitrile (PAN), polycarbonate, polysulfone, polyamide, polymethyl methacrylate (PMMA), and other membrane materials.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree