Chapter 37 Prevention of mother-to-child transmission of HIV-1
Introduction
An estimated 370,000 children were infected with HIV-1 worldwide in 2009 [1]. Although this represents a 24% decrease from 2001, over 1,000 infections continue to occur in children daily. Over 90% of these infections occurred in sub-Saharan Africa, where HIV-1 acquisition through breast milk accounts for at least 40% of infections.
In well-resourced health systems in the USA and Europe, virtual elimination of perinatal HIV-1 infection is within reach. Early identification of infection among pregnant women through routine, opt-out antenatal HIV-1 testing, immediate assessment of infected pregnant women to determine their need for treatment, and provision of antiretroviral therapy when needed or antiretroviral prophylaxis if therapy is not yet required has substantially reduced the risk of infant infection during pregnancy and delivery. When combined with elective cesarean delivery and complete avoidance of breastfeeding, these interventions have reduced MTCT rates to 1–2% [2].
However, in resource-constrained countries, where most HIV-1-infected women reside, control of the perinatal epidemic continues to be challenging. Clinical trials have identified simple, less expensive, effective antiretroviral prophylaxis regimens more relevant to these countries that can reduce transmission during pregnancy and delivery. However, implementation has been slow, as the existing limited maternal–child healthcare infrastructure has had difficulty supporting the addition of antenatal HIV-1 testing and antiretroviral programs [3]. Postnatal transmission remains a significant problem in these settings, where breastfeeding is a cornerstone of infant survival [4]. Recent clinical trials have demonstrated that antiretroviral prophylaxis of the breastfeeding infant or the lactating mother can significantly decrease postnatal HIV-1 acquisition, offering new hope for prevention in these settings [5].
Risk Factors for HIV-1 MTCT
HIV-1 can be transmitted during pregnancy, labor, and through breast milk. While different risk factors may influence transmission during each of these periods, maternal HIV-1 viral load has consistently been a strong independent predictor of transmission, regardless of timing of transmission [6, 7]. Hence, interventions that reduce viral load might be expected to influence transmission during each of these periods.
In utero and intrapartum transmission
Intrapartum transmission can occur through access of cell-free or cell-associated virus to the infant systemic circulation by maternal–fetal transfusions during uterine contractions in labor, or through the infant swallowing HIV-1 present in genital fluids during delivery, with resultant viral passage through the gastrointestinal mucosa to underlying lymphoid cells, followed by systemic dissemination. The proven efficacy of interventions restricted to the peripartum period, such as elective cesarean section performed prior to labor and rupture of membranes or antiretroviral prophylaxis administered only around the time of delivery, illustrates the importance of the intrapartum period in MTCT [8–12].
Risk factors for transmission during the antepartum and peripartum periods were examined in 3,396 infants with known infection status born between 2002 and 2006 in New York State [13]. On multivariable analysis, maternal HIV diagnosis at or after delivery, maternal acquisition of HIV during pregnancy, illicit substance use during pregnancy, having 0–2 prenatal care visits, and neonatal birth weight < 2,500 g were associated with MTCT.
Postnatal transmission
Breastfeeding substantially increases MTCT and can account for 30–50% of all transmission in breastfeeding populations. Postnatal transmission results in a significant diminution of the efficacy of antiretroviral interventions that prevent in utero and intrapartum transmission [11, 14, 15]. Thus, for optimal prevention of MTCT in a breastfeeding setting, additional interventions are needed to reduce postnatal transmission.
Determining the timing of breast milk transmission has been complicated by the difficulty in distinguishing between intrapartum and early breast milk transmission; thus, most studies have focused on postnatal transmission occurring after age 1 month. The Breastfeeding and HIV International Transmission Study was an individual patient meta-analysis involving 4,085 children in 9 clinical trials in breastfeeding populations [16]. Of 539 children with known timing of infection, 225 (42%) had late postnatal transmission (occurring after age 1 month). Overall, late postnatal transmission risk was 8.9 infections per 100 child-years of breastfeeding, with a generally constant risk throughout the breastfeeding period (~ 0.17%/week); the cumulative probability of late postnatal infection at age 18 months was 9.3%. Almost identical results were reported from the Zimbabwe Vitamin A for Mothers and Babies (ZVITAMBO) trial, which enrolled infants born to 4,495 women with chronic HIV-1 infection [17].
Although the risk of HIV-1 transmission through breast milk persists for the duration of breastfeeding, the early breastfeeding period (first 1–2 months) may be the period of highest risk. This is illustrated by the SAINT trial, in which all women and infants received effective prophylaxis against intrapartum transmission (zidovudine [AZT]/lamivudine [3TC] or single-dose nevirapine [NVP]) [18]. The trial included formula and breastfed infants; breastfeeding was the most significant risk factor for MTCT. By age 8 weeks, breastfeeding accounted for an absolute 6% increase in MTCT compared to formula-feeding. Postnatal transmission was also highest in the first few weeks of life in the control arm of the Breastfeeding and Nutrition (BAN) study (receiving single-dose NVP and 1 week postnatal AZT/3TC): postnatal transmission was 0.5%/week between 2 and 6 weeks, 0.3%/week between 7 and 12 weeks, and 0.1%/week thereafter [5, 19].
In addition to duration of breastfeeding, postnatal transmission risk factors include high plasma and maternal viral load; low CD4 count; breast milk immunologic factors; breast pathology including clinical and subclinical mastitis, nipple bleeding, cracked nipples, or breast abscess; and infant pathology that disrupts mucosal integrity, such as thrush [7, 20]. Several studies have suggested that exclusive breastfeeding is associated with lower risk of transmission than mixed feeding including breast milk and non-human milk, fluids, or other foods [20–22]. In the Zambia Exclusive Breastfeeding Study, early postnatal transmission before age 4 months was 2.7-fold higher in infants who did not exclusively breastfeed compared to those who did, adjusted for maternal CD4 count and viral load, syphilis, and low birth weight [23]. Finally, primary infection during breastfeeding has been shown to substantially increase the risk of postnatal transmission [17, 24, 25].
Antiretroviral Interventions to Prevent HIV-1 MTCT
In 1994, the PACTG 076 clinical trial first demonstrated that an antiretroviral drug, AZT, given during pregnancy, intravenously during labor, and to the newborn for 6 weeks significantly reduces in utero and intrapartum HIV-1 transmission [26]. In resource-rich countries, this relatively complex regimen was rapidly adopted as standard of care for HIV-infected pregnant women and their infants, with subsequent decline in overall population-based MTCT rates [27]. As new antiretroviral drugs became available, treatment with combination drug regimens (initially dual and then triple regimens) became standard of care, including for treatment of pregnant women. Although there were no randomized clinical trials, observational data suggested that use of combination regimens during pregnancy further reduced MTCT compared to AZT alone, at least in women requiring therapy for their own health [2, 27]. Thus, in resource-rich countries, combination regimens that include 3 or more antiretroviral drugs are currently given during pregnancy to HIV-infected women. In women who meet treatment criteria, these drugs are continued postpartum; in women who do not yet require treatment for their own health, the drugs may be discontinued postpartum.
Mechanisms of action of antiretroviral prophylaxis
Efficacy is likely multifactorial. In women with high viral loads, it is likely that lowering viral levels with antenatal antiretroviral drugs is a critical component of protection. However, antiretroviral drugs have been shown to reduce the risk of transmission even among women with very low viral levels [28]. The HIV RNA level (viral load) at delivery and receipt of antenatal antiretroviral drugs are each independently associated with transmission, suggesting that antiretroviral prophylaxis does not work solely through viral load reduction [27].
Studies have demonstrated that the quantity of cell-free and cell-associated virus in cervico-vaginal secretions is associated with MTCT, independent of plasma viral load [29]. Thus, providing prophylaxis to the infant immediately before and after extensive viral exposure during labor and delivery is an additional important mechanism of efficacy and results of clinical trials (discussed below) have demonstrated that intrapartum/postpartum antiretroviral regimens, without any maternal antenatal drug component, can significantly decrease MTCT [10–12].
Clinical trials for prevention of HIV-1 MTCT
Early international trials to prevent MTCT largely focused on antiretroviral drug use solely for prevention of transmission, without consideration of maternal treatment, because antiretroviral therapy was not generally available in resource-constrained countries at the time the studies were performed. However, treatment is now available in these settings; a key issue in decisions on which antiretroviral regimen to choose for an HIV-infected pregnant woman (or a postpartum lactating woman) is whether the drugs are being provided for treatment (in which case combination antiretroviral therapy should be provided and continued for life) or solely for MTCT prophylaxis (in which case less intensive regimens may be equally effective and antiretroviral drugs could stop when transmission risk has ceased). In resource-constrained countries, guidelines recommend that pregnant women with CD4 < 350 cells/mm3 or World Health Organization (WHO) stage III or IV disease should start on life-long combination antiretroviral therapy [30]. In a study of 3,736 HIV-1-infected pregnant women in 13 clinical programs in 8 African countries and Thailand, 52% met WHO treatment criteria and 48% did not [31]. Current research is focused on what prophylactic regimen would be most efficacious and cost-effective for the subgroup of women who don’t require therapy.
Tables 37.1–37.3 summarize results of randomized clinical trials of antiretroviral interventions for prevention of MTCT. Table 37.1 summarizes early trials of AZT, AZT/3TC, and single-dose NVP; Table 37.2 summarizes trials combining single-dose NVP with various antepartum regimens; and Table 37.3 summarizes more recent trials evaluating regimens to prevent postnatal transmission. These trials have built sequentially on each other and identified a number of simple regimens effective in reducing MTCT. Direct comparison between trials is difficult, as they enrolled patient populations from different geographic areas, infected with different viral subtypes, and having different infant feeding practices. However, some general conclusions can be drawn regarding antiretroviral drug use for prevention of MTCT that are relevant to both resource-constrained and resource-rich countries.
Table 37.1 Clinical trials of antiretroviral drugs for prevention of mother-to-child HIV-1 transmission—zidovudine, zidovudine/lamivudine, single-dose nevirapine
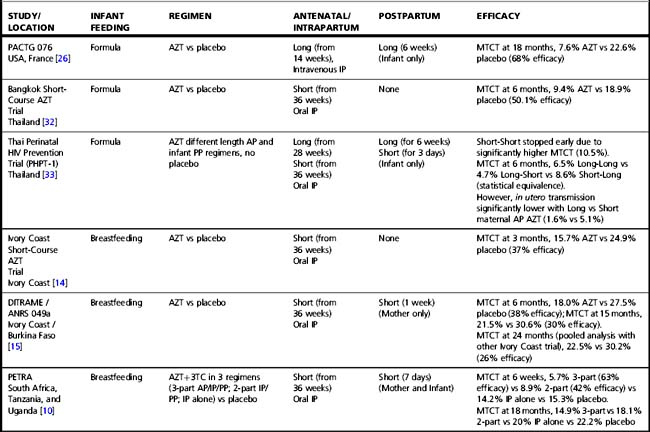
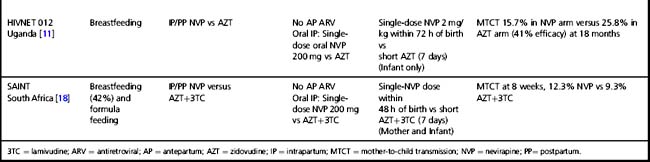
Table 37.2 Clinical trials of antiretroviral drugs for prevention of mother-to-child HIV-1 transmission—zidovudine or zidovudine/lamivudine combined with single-dose nevirapine
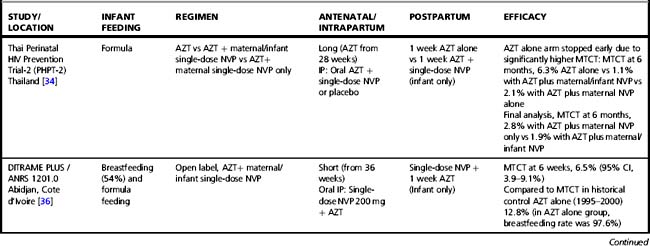
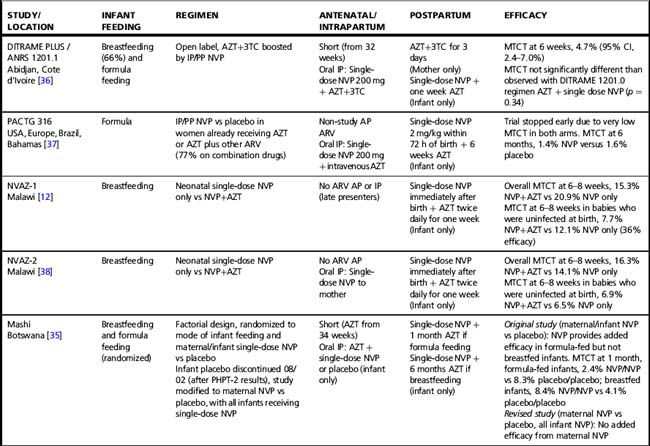
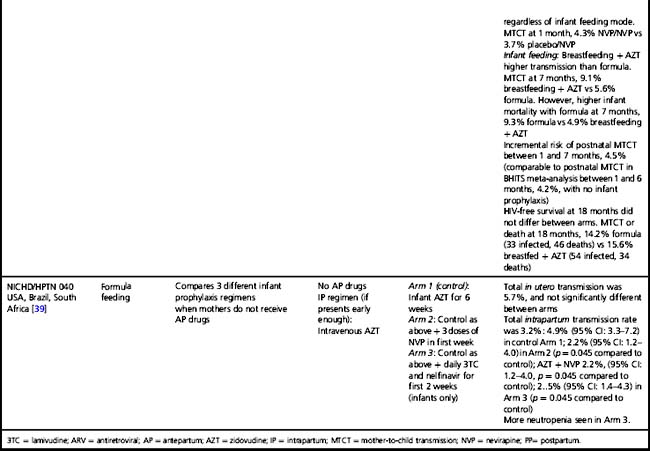
Table 37.3 Clinical trials of antiretroviral drugs for prevention of postnatal breast milk mother-to-child HIV-1 transmission
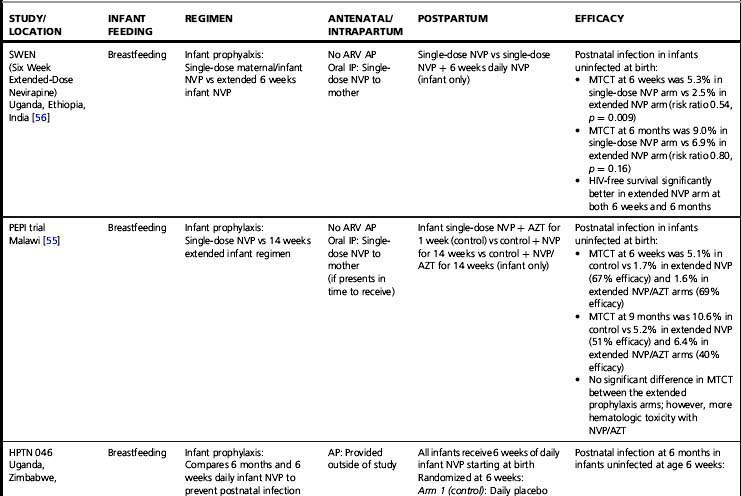
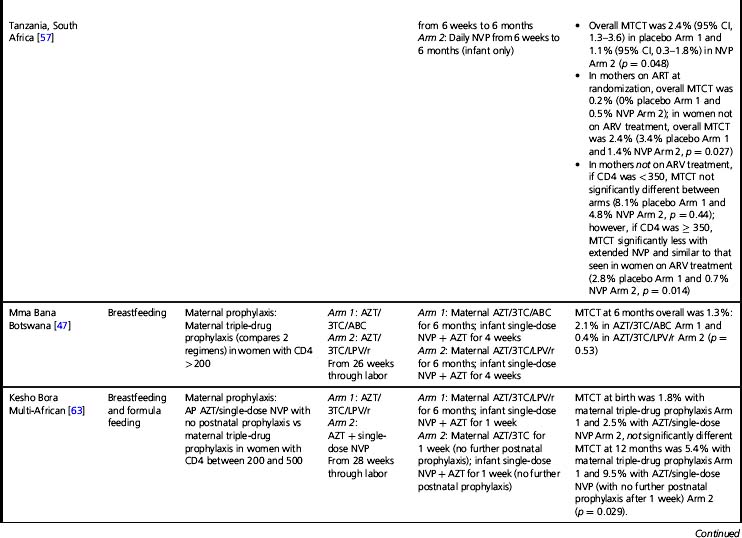
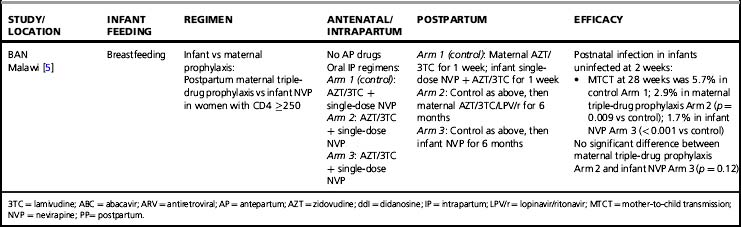
Almost all trials have included an intrapartum prophylaxis component, with varying durations of maternal antenatal and/or infant (and sometimes maternal) postpartum prophylaxis. Regimens with antenatal components starting as late as 36 weeks’ gestation and lacking infant prophylaxis can reduce transmission [32]; however, longer duration of antenatal therapy starting at 28 weeks is more effective than shorter [33]. Observational data from the European National Study of HIV in Pregnancy and Childhood have shown even longer antenatal drug duration (starting before 28 weeks’ gestation) further reduces MTCT; each additional week of drug corresponded to a 10% reduction in transmission after adjusting for viral load, mode of delivery, and infant sex [2]. More prolonged post-exposure prophylaxis of the infant does not substitute for longer duration of maternal therapy [33].
Regimens that include no antenatal prophylaxis but include intrapartum and postpartum drug administration are also effective (Table 37.1) [10, 11]. However, the PETRA study demonstrated that intrapartum pre-exposure prophylaxis alone, without continued post-exposure prophylaxis of the infant, is not effective [10]. The SAINT trial demonstrated that the two proven effective intrapartum/postpartum regimens (AZT/3TC or single-dose NVP) were similar in efficacy and safety [18].
In an attempt to improve the efficacy of short-course regimens but retain a regimen that remains appropriate to the cost limitations in resource-constrained countries, researchers evaluated whether the addition of a potent intrapartum intervention—the single-dose NVP regimen—to short-course antepartum regimens might increase efficacy (Table 37.2). In the setting of short-course AZT alone or AZT/3TC regimens, the Perinatal HIV Prevention Trial (PHPT)-2 study in non-breastfeeding women in Thailand, the Mashi study in Botswana (in the formula-fed strata), and the DITRAME studies in a partly breastfeeding population in the Cote d’Ivoire, demonstrated that the addition of single-dose NVP did significantly increase efficacy [34–36]. However, a clinical trial conducted in resource-rich countries, PACTG 316, demonstrated that the addition of single-dose NVP did not appear to offer significant benefit in the setting of potent combination antiretroviral therapy throughout pregnancy and very low viral load at the time of delivery [37]. The relative importance of the maternal and infant components of single-dose NVP remains unclear. The Thailand PHPT-2 study suggested that the infant NVP dose may not be necessary when maternal NVP is provided, and the Botswana Mashi study suggested that maternal NVP may not be necessary when infant single-dose NVP is provided at birth [34, 35].
In some countries, a significant proportion of women lack antenatal care and present to the healthcare system during labor. A trial was conducted in a breastfeeding population in Malawi to define the optimal infant prophylaxis regimen in resource-constrained settings when no antenatal maternal therapy was received (Table 37.2). The addition of one week of AZT to infant single-dose NVP reduced the risk of transmission by 36% compared to infant single-dose NVP alone (12). However, when maternal intrapartum NVP was received, thereby providing pre-exposure in addition to post-exposure prophylaxis, infant single-dose NVP alone was as effective as the combined NVP/AZT infant post-exposure prophylaxis regimen (38).
In resource-rich countries, standard infant prophylaxis in the absence of maternal antenatal antiretroviral drugs is 6 weeks of AZT. NICHD/HPTN 040 was designed to determine the optimal infant prophylaxis regimen in formula-fed infants born to women who did not receive antepartum therapy (Table 37.2). The trial compared standard 6-week infant AZT prophylaxis to 6 weeks of AZT combined with either 3 NVP doses during the first week of life or 2 weeks of nelfinavir and 3TC. Both combination regimens reduced intrapartum MTCT compared to AZT alone by approximately 50%: intrapartum MTCT was 4.9% with AZT alone versus 2.2% with AZT/NVP and 2.5% with AZT/3TC/nelfinavir [39].
In breastfeeding populations, the impact of short-course antiretroviral prophylaxis regimens on long-term risk of infant infection is diminished due to the continued transmission during the breastfeeding period. Several trials have assessed the effect of antiretroviral prophylaxis provided to the mother during lactation or to the breastfeeding infant (Table 37.3) and are reviewed in detail elsewhere [40]. It is not possible to directly compare MTCT rates in these studies. The patient populations significantly differ; all the infant prophylaxis studies except one (BAN) enrolled women regardless of CD4 count, whereas the 3 randomized trials of maternal prophylaxis restricted enrollment to women with CD4 counts > 200–250 cells/mm3 (Table 37.3). Antepartum antiretroviral drug administration and duration significantly differ: the infant prophylaxis studies enrolled women who had not received any antepartum drugs while all the maternal prophylaxis studies except one (BAN) provided antepartum drugs (of different durations, starting at 25 to 36 weeks’ gestation). Additionally, the postnatal prophylaxis duration differs between studies, with 2 infant prophylaxis studies providing only 6 to 14 weeks of postnatal prophylaxis while all of the maternal prophylaxis studies provided 6 months of postnatal prophylaxis.
Despite these differences, currently available data suggest that both infant and maternal prophylaxis are effective in reducing postnatal infection in women who don’t require therapy for their own health, and may have similar efficacy when compared during similar periods of prophylaxis [5, 40]. This is best illustrated by the BAN study, the only trial that included both infant and maternal prophylaxis arms (Table 37.3) [5]. Given two presumably similarly effective interventions, the choice of intervention to prevent MTCT for breastfeeding women who do not require treatment for their own health will involve weighing a number of different considerations, including relative costs, feasibility, and risks and benefits of the interventions.
Short- and Long-Term Safety of Antiretroviral Exposure for Infants and Women
Infant
The short-course antiretroviral regimens studied in clinical trials in resource-constrained settings have been associated with minimal short-term infant toxicity; antiretroviral exposure is associated with transient, mild hematologic abnormalities that resolve following completion of prophylaxis [41, 42]. However, longer-term outcome data in infants exposed to the more complex and prolonged maternal antiretroviral prophylaxis regimens used in resource-rich countries are still limited.
Current data indicate no increase in congenital abnormalities among offspring of women with first trimester use of most antiretroviral drugs [43]. However, there remain concerns related to efavirenz (EFV). In a primate study, prenatal EFV exposure was associated with central nervous system defects in infant monkeys and the Antiretroviral Pregnancy Registry has received six retrospective reports of central nervous system defects (e.g. meningomyelocoele) in human infants after first trimester exposure [43]. However, a meta-analysis of observational data from 1,132 women with first trimester EFV exposure from 9 prospective cohorts (including the Antiretroviral Pregnancy Registry) found no increased risk of overall birth defects compared with exposure to other antiretroviral drugs [44]. Across 11 cohorts including 1,256 live births with first trimester EFV exposure, one neural tube defect was observed, giving a prevalence of 0.08% [44]. Although these data are reassuring, the low neural tube defect incidence in the general population means that larger exposure numbers are needed to definitively rule out an increased risk of this specific defect. EFV should be avoided in the first trimester of pregnancy (although second/third trimester use may be considered). Women of childbearing potential should undergo pregnancy testing before initiating EFV therapy, and be counseled about the potential risk to the fetus and provided with adequate contraception [45].
Data are conflicting on whether combination antiretroviral drug use during pregnancy is associated with preterm delivery. A pooled analysis of 19,585 singleton births from 4 European and US observational cohorts concluded that a 3-drug regimen conferred a 1.5-fold increased adjusted odds of preterm delivery compared with a 2-drug regimen [46]. A randomized trial comparing two different combination regimens found a higher preterm delivery rate in women receiving a protease inhibitor-based compared to a triple nucleoside regimen [47]. In contrast, a US study of 777 HIV-1-infected pregnant women who were not receiving antiretroviral drugs at conception found no association of combination drugs with preterm delivery [48]. However, only 21% had received drugs during the first trimester; some studies suggest that preterm delivery is more likely to be associated with drug use early in pregnancy (e.g. first trimester or at conception) [49].
Some data suggest that short-term toxicity may be greater with combination regimens than single-drug regimens. Higher rates of anemia and neutropenia in the first few months of life were observed in uninfected infants born to mothers receiving 3-drug regimens during pregnancy compared to those exposed only to single or dual drugs, but this resolved by age 6 months [50, 51]. Additionally, uninfected infants exposed to maternal 3-drug regimens had lower birth weight and length than those exposed only to AZT, although this difference resolved (weight) or narrowed (height) by age 6 months [52].
Longer-term data are limited. Pre-clinical data indicate that some nucleoside analogue reverse transcriptase inhibitor (NRTI) drugs are carcinogenic in vitro and can be associated with mitochondrial toxicity. No increase in overall cancer risk has been observed in > 9,000 uninfected NRTI-exposed children followed to median age 5.4 years [53]. However, French researchers have reported rare occurrence of mitochondrial dysfunction in uninfected infants with in utero antiretroviral exposure, with higher risk among those exposed to combination regimens. In a cohort of 4,392 uninfected HIV-1-exposed children, evidence of mitochondrial dysfunction was identified in 12 children (with 2 deaths), yielding an 18-month incidence of 0.26% [54]. All children presented with neurologic symptoms, often with abnormal magnetic resonance imaging and/or a significant episode of hyperlactatemia, and all had an identified deficit in one of the mitochondrial respiratory chain complexes and/or abnormal muscle biopsy histology.
Extended daily infant NVP prophylaxis can prevent breast milk MTCT [5, 55–57]. Extended NVP used for up to 6 months has been studied in over 4,500 infants in the SWEN, PEPI—Malawi, HPTN 046, and BAN trials (Table 37.3); the regimen appears safe compared to control interventions, with the exception of higher number of rashes in the BAN study (however, grade 3 or 4 rash in BAN was < 2%) [5]. Daily AZT/NVP infant prophylaxis was associated with higher rates of hematologic toxicity than daily NVP [55].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


