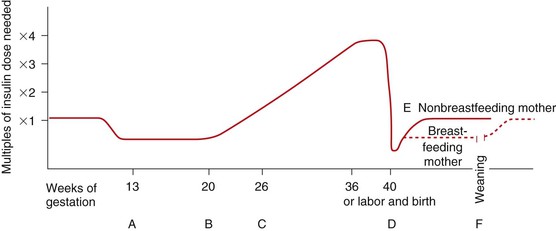
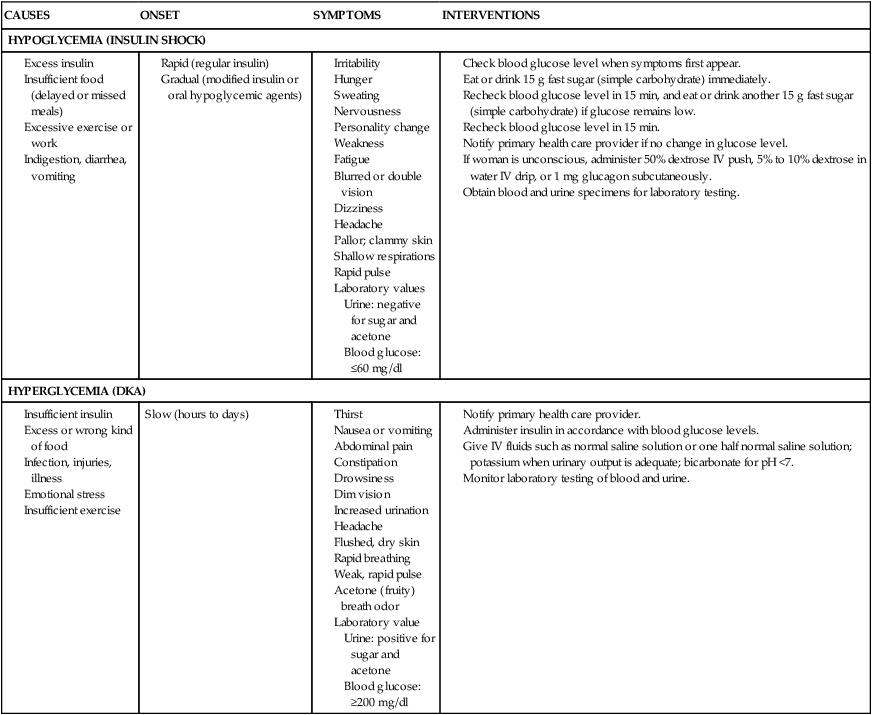
• Differentiate the types of diabetes mellitus and their respective risk factors in pregnancy. • Compare insulin requirements during pregnancy, postpartum, and with lactation. • Identify maternal and fetal risks or complications associated with diabetes in pregnancy. • Develop a plan of care for the pregnant woman with pregestational or gestational diabetes. • Explain the effects of thyroid disorders on pregnancy. • Differentiate the management for pregnant women with class I to class IV cardiac disease. • Describe the different types of anemia and their effects during pregnancy. • Explain the care of pregnant women with pulmonary disorders. • Describe the effects of neurologic disorders on pregnancy. • Outline the care of women whose pregnancies are complicated by autoimmune disorders. • Discuss the care of pregnant women who use, abuse, or are dependent on alcohol or illicit or prescription drugs. Additional related content can be found on the companion website at http://evolve.elsevier.com/Lowdermilk/Maternity/ • Case Study: Class III Cardiac Disorder • Case Study: Pregestational Diabetes • Critical Thinking Exercise: Gestational Diabetes • Nursing Care Plan: Pregnancy Complicated by Pregestational Diabetes • Nursing Care Plan: The Pregnant Woman with Heart Disease Around the world the incidence of diabetes mellitus is increasing at a rapid rate. In 2005 an estimated 20.8 million people (7% of the population) in the United States had been diagnosed with some form of diabetes. In the United States, experts predict a marked future increase in the number of women with preexisting diabetes who will become pregnant (Moore & Catalano, 2009). Diabetes mellitus is currently the most common endocrine disorder associated with pregnancy, occurring in approximately 4% to 14% of pregnant women (Gilbert, 2007). The perinatal mortality rate for well-managed diabetic pregnancies, excluding major congenital malformations, is approximately the same as for any other pregnancy (Landon, Catalano, & Gabbe, 2007). The key to an optimal pregnancy outcome is strict maternal glucose control before conception, as well as throughout the gestational period. Consequently, for women with diabetes, much emphasis is placed on preconception counseling. Pregnancy complicated by diabetes is still considered high risk. It is most successfully managed by a multidisciplinary approach involving the obstetrician, perinatologist, internist or endocrinologist, ophthalmologist, nephrologist, neonatologist, nurse, nutritionist or dietitian, and social worker, as needed. A favorable outcome requires commitment and active participation by the pregnant woman and her family. Planning the pregnancy is preferable, working before conception with the woman and her family (Landon et al., 2007). Diabetes mellitus refers to a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both (American Diabetes Association [ADA], 2008). Insulin, produced by the beta cells in the islets of Langerhans in the pancreas, regulates blood glucose levels by enabling glucose to enter adipose and muscle cells, where it is used for energy. When insulin is insufficient or ineffective in promoting glucose uptake by the muscle and adipose cells, glucose accumulates in the bloodstream, and hyperglycemia results. Hyperglycemia causes hyperosmolarity of the blood, which attracts intracellular fluid into the vascular system, resulting in cellular dehydration and expanded blood volume. Consequently, the kidneys function to excrete large volumes of urine (polyuria) in an attempt to regulate excess vascular volume and to excrete the unusable glucose (glycosuria). Polyuria, along with cellular dehydration, causes excessive thirst (polydipsia). Diabetes may be caused either by impaired insulin secretion, when the beta cells of the pancreas are destroyed by an autoimmune process, or by inadequate insulin action in target tissues at one or more points along the metabolic pathway. Both of these conditions are commonly present in the same person, and determining which, if either, abnormality is the primary cause of the disease is difficult (ADA, 2008). For additional information on diabetes, visit the American Diabetes Association’s website at www.diabetes.org. The current classification system includes four groups: type 1 diabetes, type 2 diabetes, other specific types (e.g., diabetes caused by genetic defects in B-cell function or insulin action, disease or injury of the pancreas, or drug-induced diabetes), and gestational diabetes mellitus (GDM) (ADA, 2008; Moore & Catalano, 2009). Approximately 90% of all pregnant women with diabetes have GDM (Gilbert, 2007). Of the women with pregestational diabetes, the majority (65%) of them have type 2 diabetes (Chan & Johnson, 2006). Type 1 diabetes includes cases that are caused primarily by pancreatic islet beta cell destruction and that are prone to ketoacidosis. People with type 1 diabetes usually have an abrupt onset of illness at a young age and an absolute insulin deficiency. Type 1 diabetes includes cases currently thought to be caused by an autoimmune process, as well as those for which the cause is unknown (ADA, 2008; Landon et al., 2007). Type 2 diabetes is the most prevalent form of the disease and includes individuals who have insulin resistance and usually relative (rather than absolute) insulin deficiency. Specific causes of type 2 diabetes are unknown at this time. Type 2 diabetes often goes undiagnosed for years because hyperglycemia develops gradually and is often not severe enough for the patient to recognize the classic signs of polyuria, polydipsia, and polyphagia. Most people who develop type 2 diabetes are obese or have an increased amount of body fat distributed primarily in the abdominal area. Other risk factors for the development of type 2 diabetes include aging, a sedentary lifestyle, family history and genetics, puberty, hypertension, and prior gestational diabetes. Type 2 diabetes often has a strong genetic predisposition (ADA, 2008; Moore & Catalano, 2009). GDM is any degree of glucose intolerance with the onset or first recognition occurring during pregnancy. This definition is appropriate whether or not insulin is used for treatment or the diabetes persists after pregnancy. It does not exclude the possibility that the glucose intolerance preceded the pregnancy or that medication might be required for optimal glucose control. Women experiencing gestational diabetes should be reclassified 6 weeks or more after the pregnancy ends (ADA, 2008; Moore & Catalano, 2009). Dr. Priscilla White, a physician who worked with pregnant women with diabetes during the 1940s, developed a classification system specifically for use with this group of women (Table 20-1). Dr. White’s system was based on age at diagnosis, duration of illness, and presence of vascular disease (Landon et al., 2007; Moore & Catalano, 2009). Her classification system has been modified through the years but is still frequently used today to assess both maternal and fetal risk. Women in classes A through C generally have good pregnancy outcomes as long as their blood glucose levels are well controlled. Women in classes D through T, however, usually have poorer pregnancy outcomes because they have already developed the vascular damage that often accompanies long-standing diabetes. TABLE 20-1 White’s Classification of Diabetes in Pregnancy (Modified) OGTT, Oral glucose tolerance test. Sources: Landon, M., Catalano, P., & Gabbe, S. (2007). Diabetes mellitus complicating pregnancy. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone; Moore, T., & Catalano, P. (2009). Diabetes in pregnancy. In R. Creasy, R. Resnik, & J. Iams (Eds.), Creasy and Resnik’s maternal-fetal medicine: Principles and practice (6th ed.). Philadelphia: Saunders. During the first trimester of pregnancy the pregnant woman’s metabolic status is significantly influenced by the rising levels of estrogen and progesterone. These hormones stimulate the beta cells in the pancreas to increase insulin production, which promotes increased peripheral use of glucose and decreased blood glucose, with fasting levels being reduced by approximately 10% (Fig. 20-1, A). At the same time, an increase in tissue glycogen stores and a decrease in hepatic glucose production occur, which further encourage lower fasting glucose levels. As a result of these normal metabolic changes of pregnancy, women with insulin-dependent diabetes are prone to hypoglycemia during the first trimester. During the second and third trimesters, pregnancy exerts a “diabetogenic” effect on the maternal metabolic status. Because of the major hormonal changes, decreased tolerance to glucose, increased insulin resistance, decreased hepatic glycogen stores, and increased hepatic production of glucose occur. Rising levels of human chorionic somatomammotropin, estrogen, progesterone, prolactin, cortisol, and insulinase increase insulin resistance through their actions as insulin antagonists. Insulin resistance is a glucose-sparing mechanism that ensures an abundant supply of glucose for the fetus. Maternal insulin requirements gradually increase from approximately 18 to 24 weeks of gestation to approximately 36 weeks of gestation. Maternal insulin requirements may double or quadruple by the end of the pregnancy (Fig. 20-1, B and C). At birth, expulsion of the placenta prompts an abrupt drop in levels of circulating placental hormones, cortisol, and insulinase (Fig. 20-1, D). Maternal tissues quickly regain their prepregnancy sensitivity to insulin. For the nonbreastfeeding mother the prepregnancy insulin-carbohydrate balance usually returns in approximately 7 to 10 days (Fig. 20-1, E). Lactation uses maternal glucose; therefore the breastfeeding mother’s insulin requirements will remain low during lactation. On completion of weaning the mother’s prepregnancy insulin requirement is reestablished (Fig. 20-1, F). Approximately 2 per 1000 pregnancies are complicated by preexisting diabetes. Women who have pregestational diabetes may have either type 1 or type 2 diabetes, which may or may not be complicated by vascular disease, retinopathy, nephropathy, or other diabetic sequelae. Type 2 is the more common diagnosis compared with type 1. Almost all women with pregestational diabetes are insulin dependent during pregnancy. According to White’s classification system, these women fall into classes B through T (see Table 20-1). Preconception counseling is recommended for all women of reproductive age who have diabetes because it is associated with less perinatal mortality and fewer congenital anomalies (Moore & Catalano, 2009). Under ideal circumstances, women with pregestational diabetes are counseled before the time of conception to plan the optimal time for pregnancy, establish glycemic control before conception, and diagnose any vascular complications of diabetes. However, estimates indicate that fewer than 20% of women with diabetes in the United States participate in preconception counseling (Landon et al., 2007). Although maternal morbidity and mortality rates have improved significantly, the pregnant woman with diabetes remains at risk for the development of complications during pregnancy. Poor glycemic control around the time of conception and in the early weeks of pregnancy is associated with an increased incidence of miscarriage. Women with good glycemic control before conception and in the first trimester are no more likely to miscarry than women who do not have diabetes (Moore & Catalano, 2009). Poor glycemic control later in pregnancy, particularly in women without vascular disease, increases the rate of fetal macrosomia. Macrosomia has been defined in several different ways, including a birthweight more than 4000 to 4500 g, birthweight above the 90th percentile, and estimates of neonatal adipose tissue. Macrosomia occurs in approximately 40% of pregestational diabetic pregnancies and in up to 50% of pregnancies complicated by GDM (Landon et al., 2007; Moore & Catalano, 2009). Infants born to mothers with diabetes tend to have a disproportionate increase in shoulder, trunk, and chest size. Because of this tendency the risk of shoulder dystocia is greater in these babies than in other macrosomic infants. Women with diabetes therefore face an increased likelihood of cesarean birth because of failure of fetal descent or labor progress or of operative vaginal birth (birth involving the use of episiotomy, forceps, or vacuum extractor) (Landon et al.; Moore & Catalano). Women with preexisting diabetes are at risk for several obstetric and medical complications. In general the risk of developing these complications increases with the duration and severity of the woman’s diabetes. In one study the rates of preeclampsia, preterm birth, cesarean birth, and maternal mortality were much higher in women with preexisting diabetes than in women who did not have this disease. Approximately a third of women who have had diabetes for more than 20 years, for example, develop preeclampsia. Women with nephropathy and hypertension in addition to diabetes are also increasingly likely to develop preeclampsia. The rate of hypertensive disorders in all types of pregnancies complicated by diabetes is 15% to 30%. Chronic hypertension occurs in 10% to 20% of all diabetic pregnancies, and in up to 40% of those in women who have preexisting renal or retinal vascular disease (Moore & Catalano, 2009). Hydramnios (polyhydramnios) occurs approximately 10 times more often in diabetic than in nondiabetic pregnancies. Hydramnios (amniotic fluid in excess of 2000 ml) is associated with premature rupture of membranes, onset of preterm labor, and postpartum hemorrhage (Cunningham, Leveno, Bloom, Hauth, Gilstrap, & Wenstrom, 2005). Ketoacidosis (accumulation of ketones in the blood resulting from hyperglycemia and leading to metabolic acidosis) occurs most often during the second and third trimesters, when the diabetogenic effect of pregnancy is the greatest. When the maternal metabolism is stressed by illness or infection, the woman is at increased risk for diabetic ketoacidosis (DKA) DKA can also be caused by poor patient compliance with treatment or the onset of previously undiagnosed diabetes (Moore & Catalano, 2009). The use of beta-mimetic drugs such as terbutaline for tocolysis to arrest preterm labor may also contribute to the risk for hyperglycemia and subsequent DKA (Cunningham et al., 2005; Iams, Romero, & Creasy, 2009). DKA may occur with blood glucose levels barely exceeding 200 mg/dl, as compared with 300 to 350 mg/dl in the nonpregnant state. In response to stress factors such as infection or illness, hyperglycemia occurs as a result of increased hepatic glucose production and decreased peripheral glucose use. Stress hormones, which act to impair insulin action and further contribute to insulin deficiency, are released. Fatty acids are mobilized from fat stores to enter into the circulation. As they are oxidized, ketone bodies are released into the peripheral circulation. The woman’s buffering system is unable to compensate, and metabolic acidosis develops. The excessive blood glucose and ketone bodies result in osmotic diuresis with subsequent loss of fluid and electrolytes, volume depletion, and cellular dehydration. DKA is a medical emergency. Prompt treatment is necessary to prevent maternal coma or death. Ketoacidosis occurring at any time during pregnancy can lead to intrauterine fetal death. The incidence of DKA during pregnancy has decreased to approximately 2% from a rate of 20% or more in the past. The rate of intrauterine fetal demise (IUFD) with DKA, formerly approximately 35%, is currently 10% or less (Moore & Catalano, 2009) (Table 20-2). TABLE 20-2 Differentiation of Hypoglycemia (Insulin Shock) and Hyperglycemia (Diabetic Ketoacidosis) The risk of hypoglycemia (a less-than-normal amount of glucose in the blood) is also increased. Early in pregnancy, when hepatic production of glucose is diminished and peripheral use of glucose is enhanced, hypoglycemia occurs frequently, often during sleep. Later in pregnancy, hypoglycemia may also result as insulin doses are adjusted to maintain euglycemia (a normal blood glucose level). Women with a prepregnancy history of severe hypoglycemia are at increased risk for severe hypoglycemia during gestation. Mild to moderate hypoglycemic episodes do not appear to have significant deleterious effects on fetal well-being (see Table 20-2). Despite the improvements in care of pregnant women with diabetes, intrauterine fetal demise (IUFD) (sometimes known as stillbirth) is still a major concern. Approximately 2% to 5% of all fetal deaths occur in women whose pregnancies are complicated by preexisting diabetes. Hyperglycemia, ketoacidosis, congenital anomalies, infections, and maternal obesity are thought to be reasons for fetal death. In the third trimester, fetal acidosis is the most likely cause of fetal death (Paidas & Hossain, 2009). The most important cause of perinatal loss in diabetic pregnancy is congenital malformations, which account for 30% to 50% of all perinatal loss (Lindsay, 2006). The incidence of congenital malformations is related to the severity and duration of the diabetes. Hyperglycemia during the first trimester of pregnancy, when organs and organ systems are forming, is the main cause of diabetes-associated birth defects. Anomalies commonly seen in infants affect primarily the cardiovascular system, the CNS, and the skeletal system (Cunningham et al., 2005; Moore & Catalano, 2009) (see Chapter 24). The fetal pancreas begins to secrete insulin at 10 to 14 weeks of gestation. The fetus responds to maternal hyperglycemia by secreting large amounts of insulin (hyperinsulinism). Insulin acts as a growth hormone, causing the fetus to produce excess stores of glycogen, protein, and adipose tissue and leading to increased fetal size, or macrosomia. Birth injuries are more common in infants born to mothers with diabetes compared with mothers who do not have diabetes and macrosomic fetuses have the highest risk for this complication. Common birth injuries associated with diabetic pregnancies include brachial plexus palsy, facial nerve injury, humerus or clavicle fracture, and cephalhematoma. Most of these injuries are associated with difficult vaginal birth and shoulder dystocia (Moore & Catalano, 2009). Hypoglycemia at birth is also a risk for infants born to mothers with diabetes (for further discussion of neonatal complications related to maternal diabetes, see Chapter 24).
Pregnancy at Risk
Preexisting Conditions
Web Resources
![]()
Metabolic Disorders

Diabetes Mellitus
Pathogenesis
Classification
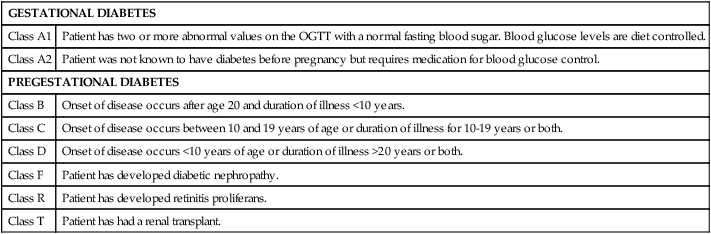
White’s classification of diabetes in pregnancy.
GESTATIONAL DIABETES
Class A1
Patient has two or more abnormal values on the OGTT with a normal fasting blood sugar. Blood glucose levels are diet controlled.
Class A2
Patient was not known to have diabetes before pregnancy but requires medication for blood glucose control.
PREGESTATIONAL DIABETES
Class B
Onset of disease occurs after age 20 and duration of illness <10 years.
Class C
Onset of disease occurs between 10 and 19 years of age or duration of illness for 10-19 years or both.
Class D
Onset of disease occurs <10 years of age or duration of illness >20 years or both.
Class F
Patient has developed diabetic nephropathy.
Class R
Patient has developed retinitis proliferans.
Class T
Patient has had a renal transplant.
Metabolic changes associated with pregnancy
Pregestational Diabetes Mellitus
Preconception counseling
Maternal risks and complications
Fetal and neonatal risks and complications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pregnancy at Risk: Preexisting Conditions
Get Clinical Tree app for offline access
