Chapter 24 Pneumocystis pneumonia
Epidemiology
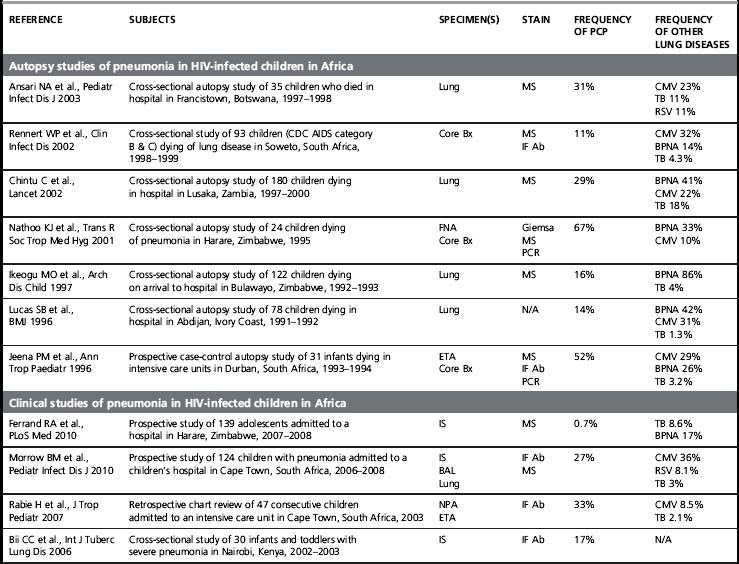
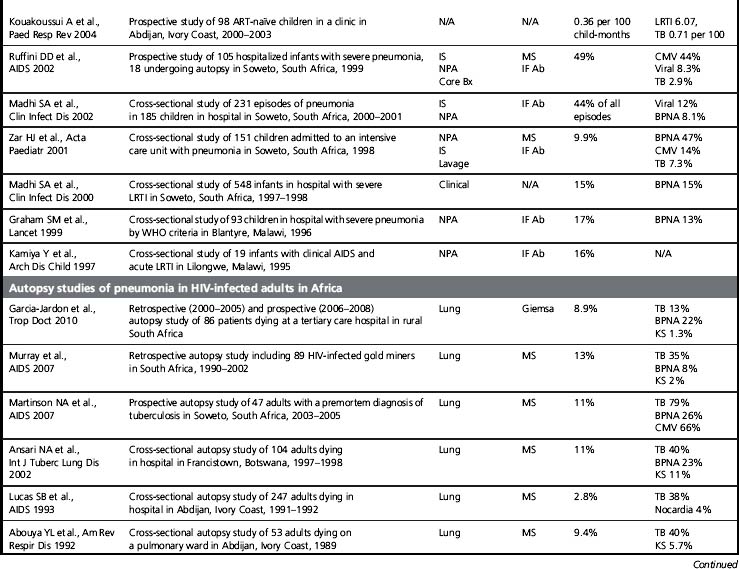
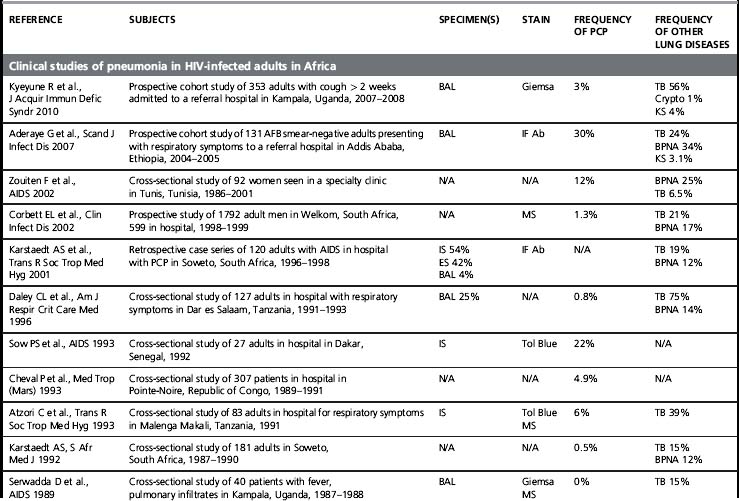
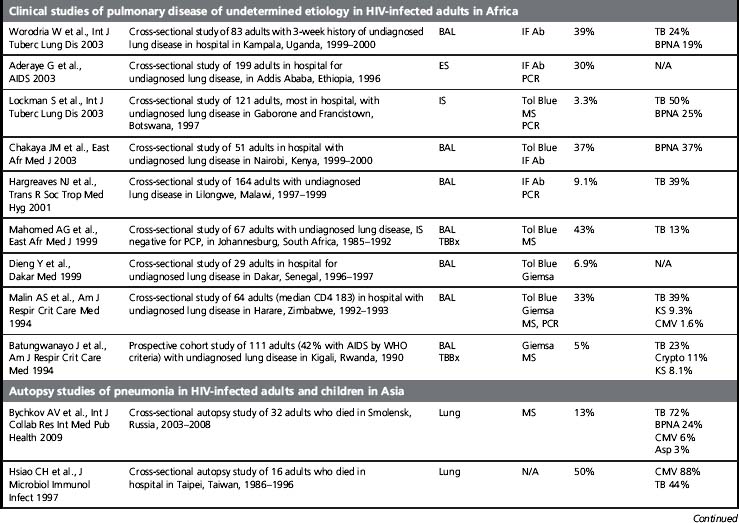
Although its incidence has declined dramatically, PCP remains a leading AIDS-defining opportunistic infection in the USA and in the countries that comprise the World Health Organization (WHO) Western European Region [1]. PCP is also a frequent opportunistic infection in HIV-infected patient cohorts, and it is an important cause of HIV-associated mortality [2]. Of concern, PCP is described in Africa, Asia, and Latin America, regions of the world where > 90% of the estimated people living with HIV/AIDS reside and where access to combination antiretroviral therapy and even PCP prophylaxis with trime thoprim-sulfamethoxazole (TMP/SMX) is more limited (Table 24.1). At present, most of the data on PCP in these regions is limited to single institution studies [3]. These studies include different study designs, study populations, diagnostic procedures, and specific criteria for defining PCP (clinical versus microscopic confirmation) that limit firm conclusions. Nevertheless, PCP appears to be a significant opportunistic infection in HIV-infected people throughout the world. Importantly, a substantial proportion of these HIV-infected patients with PCP have been found to have concurrent tuberculosis, a finding that complicates the diagnosis and treatment [4]. As the HIV/AIDS epidemic progresses in these regions, it is increasingly important to develop diagnostic and treatment algorithms that can be used in settings where resources, diagnostic tests, and medications are limited.
Risk Factors for Pneumocystis Pneumonia
A CD4 count < 200 cells/mm3, a history of oropharyngeal candidiasis, and prior PCP are all well-established risk factors for PCP [5]. PCP was the first disease to illustrate the role of the CD4-lymphocyte count as a tool for assessing the risk for the development of an HIV-associated opportunistic infection [6]. Several studies demonstrate that the risk of PCP is increased in persons with a CD4 count < 200 cells/mm3 and that the majority of PCP cases occur in patients whose CD4 count is < 200 cells/mm3 [6, 7]. Thus, a CD4 count that is significantly > 200 cells/mm3 argues against the presence of PCP. Unfortunately, CD4-lymphocyte determination is unavailable in some parts of the world, and clinicians are left to infer the degree of immunocompromise from indirect measures such as the absolute lymphocyte count.
Clinical Features
The clinical presentation of PCP in HIV-infected persons differs from the presentation in other immunocompromised persons. In general, HIV-infected patients present with a subacute onset and longer symptom duration than other immunocompromised patients [8]. Compared with HIV-negative patients, HIV-infected patients with PCP present with a higher arterial oxygen tension and a lower alveolar-arterial oxygen gradient. In addition, bronchoscopy with bronchoalveolar lavage (BAL) examination reveals significantly greater numbers of Pneumocystis organisms and fewer neutrophils in HIV-infected patients [9].
Symptoms and Signs
Classically, patients with PCP present with fever, cough, and dyspnea of 2–4 weeks, duration [10]. The cough is usually dry and non-productive unless a concurrent bacterial infection is present. Fatigue is a frequent complaint, whereas chest pain (unless due to an associated pneumothorax), chills, and night sweats are less common. Symptoms are often subtle at the onset but are gradually progressive and may be present for weeks and occasionally months before diagnosis.
Laboratory Tests
No current laboratory test is specific for PCP. The serum lactate dehydrogenase (LDH) is usually elevated in patients with PCP [11]. Published studies report the sensitivity of an elevated serum LDH for PCP to range from 82 to 100%. Unfortunately, the serum LDH is a non-specific test, and elevations are seen in many pulmonary and non-pulmonary conditions. The arterial blood gas (ABG) is an essential laboratory test in patients with PCP. A patient with PCP will usually have a decreased arterial oxygen tension, an increased alveolar-arterial oxygen gradient, and a respiratory alkalosis. The ABG should be used to decide whether to admit a patient to the hospital to receive supplemental oxygen, whether to administer adjunctive corticosteroids (PaO2 < 70 mmHg or an alveolar-arterial oxygen gradient > 35 mmHg), and to assess response to PCP therapy.
Chest Radiograph
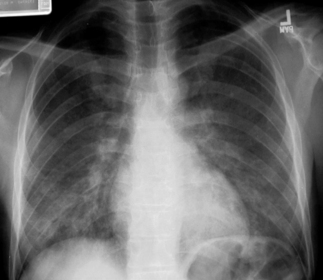
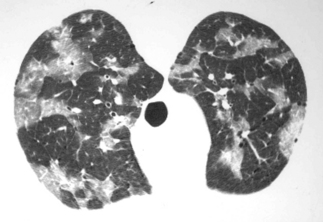
The chest radiograph is the cornerstone of the diagnostic evaluation of patients with suspected PCP. Classically, patients with PCP present with bilateral, diffuse, symmetric reticular (interstitial) or granular opacities on their chest radiograph (Fig. 24.1) [12]. PCP often begins with central or perihilar opacities and a middle-lower lung zone predominance. As with the classic radiographic presentation, these opacities are bilateral and symmetric and can progress to diffuse involvement if the disease is left undiagnosed and untreated. However, patients with PCP can occasionally present with unilateral, focal, or asymmetric opacities on their chest radiograph, and the specific pattern seen is often more important than the exact distribution. Thus, PCP must be considered in any HIV-infected patient who is at risk for PCP, has a compatible clinical presentation, and presents with reticular or granular opacities on chest radiograph, regardless of whether the findings are unilateral or bilateral, focal or diffuse, asymmetric or symmetric. Clearly though, the presence of bilateral, diffuse, and symmetric reticular (interstitial) or granular opacities increases the probability of PCP significantly. In one study, patients who had interstitial infiltrates noted on their radiograph had a 4.4 greater odds of having PCP than those without this pattern, and patients who had interstitial infiltrates that involved five or six of the six defined lung zones (upper, middle, and lower lung zones on the right and left lungs) had a 5.3 greater odds of having PCP [13].
Thin-walled cysts or pneumatoceles were reported in 7% of PCP cases in one large radiology series [12]. Pneumatoceles may be present at the time of diagnosis, may develop while on therapy, and may persist despite successful therapy. The pneumatoceles may be single or multiple in number and small or large in size. Usually, pneumatoceles are multiple in number and located in the upper lobes. Importantly, the presence of pneumatoceles predisposes patients to the development of pneumothorax, a difficult management problem for clinicians caring for PCP patients. However, pneumothoraces may also occur in the absence of radiographically demonstrable pneumatoceles.
There are a few studies on the course of respiratory symptoms and chest radiographic abnormalities in patients successfully treated for PCP [12, 14]. Patients with PCP often experience a transient clinical and radiographic worsening in the first 3–5 days of PCP therapy. In one study of 104 patients with PCP, 46% experienced a deterioration in radiographic findings at 1 week [12]. In this same study, approximately one-third of the patients showed no change in their radiograph over the first 3 weeks. A general rule is that the more severe the PCP, the more prolonged the time to clinical and chest radiographic resolution.
Other Tests
Tests including high-resolution computed tomography (HRCT) of the chest, typically demonstrating ground-glass opacities (Fig. 24.2), pulmonary function tests demonstrating decreased diffusing capacity for carbon monoxide (DLCO), and gallium scanning showing increased pulmonary uptake can all be used in the diagnostic evaluation of patients with suspected PCP [11]. Typically, these studies are performed in patients whose clinical presentation is strongly suggestive of PCP but whose chest radiograph is normal or minimally abnormal. However, these tests are relatively expensive and typically unavailable in many parts of the world. One simple test for PCP is non-invasive exercise oximetry, since patients with PCP will often have a decline in their oxygen saturation with exertion.
Diagnosis
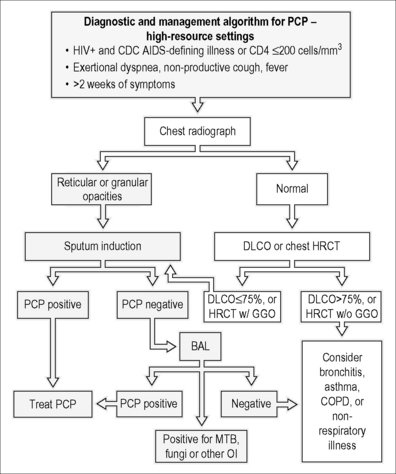
There is no universally agreed upon approach to the diagnostic evaluation of suspected PCP. Some institutions treat patients with suspected PCP empirically, while others pursue a definitive microscopic diagnosis (Fig. 24.3) [15]. In patients with suspected PCP, it is generally advantageous to make a definitive diagnosis. A false presumptive diagnosis of PCP may lead to failure or delay in making the correct diagnosis. Additionally, the drugs used to treat PCP have significant side effects, incurring unnecessary risk in the PCP-negative patient (see Treatment below). As Pneumocystis cannot be routinely cultured, the diagnosis of PCP traditionally relies on microscopic visualization of the characteristic cysts and/or trophic forms on stained respiratory specimens. Typically, these respiratory specimens are obtained from sputum induction or bronchoscopy. However, institutions report different degrees of success with sputum induction and bronchoscopy with BAL with and without transbronchial biopsiy (TBBx). Advances in polymerase chain reaction (PCR) technology have enabled the use of rapid, non-invasive procedures such as oropharyngeal washing as a method for specimen acquisition. In one study, a non-invasive, 60-s gargle (oropharyngeal wash) specimen paired with a PCR-based quantitative assay had a sensitivity of 88% and specificity of 85% [16]. However, in the absence of prospective studies comparing various management and diagnostic strategies, the specific approach to a patient with suspected PCP is often based on the prevalence of PCP, clinician and institutional preferences and experiences, and availability of diagnostic procedures and microbiologic techniques and assays. In areas of the world where tuberculosis is prevalent and where resources are limited, the examination of sputum for acid-fast bacilli may be a prudent diagnostic and public health practice (Fig. 24.4). Studies designed to examine practical, cost-effective diagnostic approaches to the patient with suspected PCP in a resource-limited setting are needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree