Chapter 23 Physiological changes during labour
Learning outcomes for this chapter are:
1. To outline the changes involved in myometrial activation and cervical ripening
2. To discuss the physiological factors that may be responsible for initiating labour
3. To outline the role of hormones in the maintenance of labour
4. To describe the physiology underlying the three stages of labour, including the physiology of effective uterine contractions, the mechanism of the second stage and the steps involved in placental separation in the third stage
5. To discuss physiological changes that occur to other systems to support labour
6. To describe fetal responses and identify measures of fetal wellbeing during labour.
contraction-associated proteins (CAPs)
corticotropin-releasing hormone (CRH)
dehydro-epiandrosterone sulfate (DHEAS)
partial pressure of carbon dioxide in the arterial circulation (PaCO2)
partial pressure of oxygen in the arterial circulation (PaO2)
These processes are the focus of this chapter. Additional readings are provided at the end of the chapter for a more in-depth exploration of specific topics.
PHYSIOLOGY OF LABOUR
Uterine changes in preparation for labour
The myometrium consists of bundles of non-striated muscle fibres, intermixed with connective tissue, blood and lymph vessels and nerves. Throughout pregnancy the uterus is preparing for labour and there is an enormous increase in the bulk of the myometrium due to hypertrophy and hyperplasia of the cells. Myometrial cells are smooth muscle cells and therefore are capable of spontaneous activity independent of external stimuli. Low-amplitude activity occurs even in a non-pregnant uterus (Challis 2001). During pregnancy, however, contractions are largely prevented by uterotonic inhibitors, such as progesterone, nitric oxide and prostacyclin, and those that do occur tend to be mild, irregular and non-synchronised. Women are generally not aware of these contractions. As pregnancy progresses, contractions gradually increase in intensity and frequency until, in labour, strong, synchronous, effective contractions occur.
Changes in the structure of myometrial cells enable them to contract more strongly and to maintain this through labour. The initial changes are termed ‘activation’. Four main events occur during activation: the ratio of hormones changes, electrical activity increases, myometrial cells become more responsive, and there is an increase in numbers of ion channels. This occurs in the last few weeks of pregnancy as levels of uterotonic inhibitors decrease and those of oestrogen and contraction-associated proteins (CAPs) increase. CAPs include gap-junction proteins, myometrial oxytocin receptors and prostaglandin receptors, and calcium and sodium channels (Blackburn 2007). Increased gap-junction formation facilitates the spread of electrical activity over the myometrium, and increased numbers of prostaglandin and oxytocin receptors enhance the responsiveness of target tissues to these uterotonins. Increased numbers of sodium ion channels facilitate sodium movement into the cells; this is required for electrical excitation. Increased numbers of calcium ion channels enable increased calcium movement into the cells; calcium is required for binding of myosin to actin during contraction.
Cervical changes in preparation for labour
Another region of the uterus that undergoes changes is the cervix. During pregnancy, the cervix serves as a protective barrier from invading microorganisms and it is also important in retaining the fetus in the uterus (Word et al 2007). Stretching of the cervix is resisted due to its high content of connective tissue, which is made up of collagen-fibre bundles embedded in a proteoglycan matrix.
Before effacement and dilation can occur, the cervix must change structure and soften or ‘ripen’, and this occurs towards the end of pregnancy. Results from studies using serial measurements of cervical length indicate that the process of cervical ripening precedes myometrial contractions of labour by several weeks (Word et al 2007). The softening process is characterised by an infiltration of leucocytes, an increase in water and a decrease in collagen content of the cervix. Infiltration of the cervix by leucocytes and macrophages leads to the release of proteolytic enzymes that cause collagen degradation. Increases in hyaluronic acid levels and changes in blood vessel permeability increase the water content of the cervix. Increases in levels of glycoaminoglycans reduce the ability of collagen fibres to bind together (Johnson 2007; Winkler et al 1999). The changes associated with cervical ripening are independent of myometrial contractions (Blackburn 2007).
The process of cervical ripening is complex and depends on the hormones present at the end of pregnancy. Increases in oestrogen relative to progesterone and increases in relaxin levels promote collagen degradation. Nitric oxide levels in the cervix increase near term and may act with prostaglandin E2 to induce vasodilation and facilitate neutrophil infiltration (Ledingham et al 2000). Stretching of the cervix results in the local release of prostaglandin F2α (PGF2α) and the release of oxytocin from the posterior pituitary (the Ferguson reflex), which in turn increase uterine activity (Blackburn 2007). Once the cervix is prepared physiologically and structurally, labour can begin.
WHAT INITIATES LABOUR?
In a number of mammalian species, the onset of labour is timed primarily by the fetus via secretions of the adrenal cortex which increase oestrogen levels and subsequently PGF2α in the uterus. PGF2α activates
Clinical point
Prostaglandin E2 (PGE2), administered vaginally, may be used to ripen the cervix or for induction of labour. It is associated with lower rates of failed induction and higher rates of delivery within a reasonable interval than amniotomy and/or oxytocin. There are a wide range of doses and formulations of vaginal PGE2, with various gels, tablets, pessaries and slow-release inserts, and currently there is no evidence for the superiority of one PGE2 preparation over another (Keirse 2006).
myometrial contractions and cervical ripening (Johnson 2007). In humans, it is likely that maternal as well as fetal mechanisms are involved, although the final pathway appears to involve activation of the fetal hypothalamic–pituitary–adrenal axis (Norwitz et al 1999).
It is known that increases in placental uterotonic hormones are linked to maturation of the fetal adrenal glands and the mechanical stretching of the uterus due to the growing fetus. Maternal adrenal glands and other endocrine or neural mechanisms may also play a role (Johnson 2007).
A growing number of placental hormones have been identified although their exact function in labour is not yet clear (Reis et al 2001). The roles of some of the key hormones and chemical mediators that contribute to labour, such as corticotrophin-releasing hormone (CRH), prostaglandins (PGs), oxytocin, oestrogen, progesterone, relaxin and nitric oxide (NO), are outlined below.
Corticotrophin-releasing hormone
It is suggested that placental CRH plays an important role in the onset of labour (Challis 2001; Grammatopoulos 2008; Grammatopoulos & Hillhouse 1999; Torricelli et al 2007), along with maturation of the fetal hypothalamic–pituitary–adrenal system. CRH is a neuropeptide produced by the hypothalamus, placenta, decidua, chorion and amnion. CRH receptors are found in the decidua and fetal membranes and in the myometrium. The complexity of control is highlighted by the fact that CRH appears to have a dual role: during pregnancy it promotes uterine quiescence; during labour, under the influence of oxytocin, it binds to different receptor types and promotes uterine contraction (Grammatopoulos 2008).
There is strong evidence for the role of CRH in the initiation of labour. For example, placental CRH levels increase markedly after 35 weeks gestation, partly due to a 60% decrease in protein binding of the hormone, and then CRH levels decrease by 50% within 20–30 minutes of birth. Longer duration of labour has been associated with lower levels of CRH (Chrousos, cited in Blackburn 2007). Additionally, CRH levels are increased in preterm labour (Torricelli et al 2007) and in pregnancies complicated by pregnancy-induced hypertension and intrauterine growth restriction (Petraglia, cited in Grammatopoulos & Hillhouse 1999) which may lead to preterm labour.
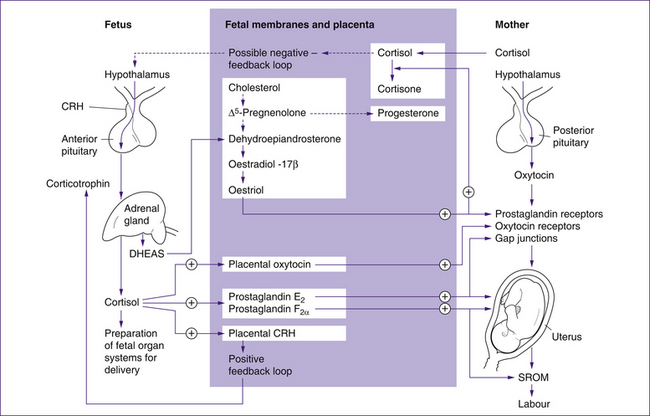
Grammatopoulos and Hillhouse (1999) proposed that placental CRH initiates labour via mechanisms that increase production of placental oestriol (active form of oestrogen) and fetal cortisol. The pathways for this are shown in Figure 23.1. CRH stimulates the fetal adrenal gland to produce cortisol and the oestrogen precursor dehydroepiandrosterone sulfate (DHEAS). Fetal cortisol exerts positive feedback to increase placental CRH levels, and increases prostaglandin and oxytocin synthesis. DHEAS is an essential precursor to placental oestrogen synthesis, particularly oestriol, which drives other mechanisms associated with the initiation of labour. The specialised fetal adrenal cells responsible for DHEAS production atrophy after birth.
Although cortisol appears to have a role in the initiation of labour, some evidence suggests that labour may still progress when levels of fetal cortisol are low. For example, neither fetal anencephaly, where there is an absence of adrenocorticotrophic hormone (ACTH), nor adrenal hypoplasia, where cortisol production is reduced, necessarily result in prolonged labour (Johnson 2007). Other clinical evidence indicates that although maternal corticosteroid administration results in a decrease of fetoplacental oestrogen production, it does not delay the onset of labour (Johnson 20007) as would be expected.
In summary, placental CRH behaves differently to hypothalamic CRH. Placental CRH may promote uterine quiescence during pregnancy and then enhance contractility during labour. It is suggested that, via the production of fetal cortisol and DHEAS, CRH leads to increased oestrogen production in the placenta, which drives the other mechanisms of labour such as an increase in numbers of oxytocin receptors and gap junctions and levels of prostaglandins.
Prostaglandins
Prostaglandins are lipids that are synthesised in tissues throughout the body. They are local hormones (paracrine hormones), which act at or near the place where they are synthesised. Prostaglandins are uterotonins directly responsible for uterine contraction. Two prostaglandins are particularly important in labour: PGE2 and PGF2α. These prostaglandins stimulate smooth muscle fibres to contract, stimulate the formation of gap junctions in myometrial tissue, and increase calcium levels in myometrial cells. As mentioned previously, prostaglandins also have a role in softening the cervix, enabling effacement and dilation (Johnson 2007; Khan et al 2008).
Levels of PGE2 and PGF2α in the amniotic fluid are known to increase before and during labour; however, evidence for an increase of these hormones in maternal plasma before labour begins is not conclusive (Johnson 2007).
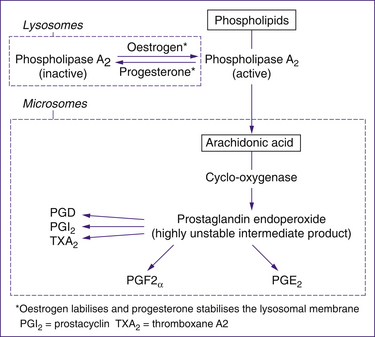
Prostaglandin synthesis has a complex pathway, outlined in Figure 23.2. During labour, prostaglandins are synthesised by the decidua, cervix, placenta and fetal membranes. Arachidonic acid is the common precursor of both PGE2 and PGF2α. Arachidonic acid is produced by the action of phospholipase A2 on phospholipids in the fetal membranes and the decidua. Phospholipase A2 is stored within lysosomes, which release the enzyme in the presence of oestrogen, and retain the enzyme in the presence of progesterone. Consequently, a rise in the oestrogen-to-progesterone ratio results in increased phospholipase A2, leading to increased production of arachidonic acid and prostaglandin synthesis. Oxytocin also promotes prostaglandin release.
Other evidence that prostaglandins contribute to the initiation of labour comes from the finding that inhibitors of prostaglandin synthesis, such as the anti-inflammatory drug indomethacin, are effective at reducing preterm labour (Enkin et al 2000; Khan et al 2008).
Oxytocin
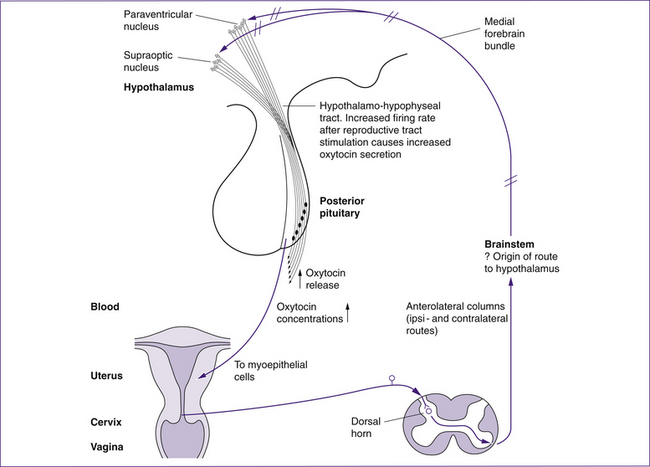
Another hormone essential for labour is oxytocin. Oxytocin is released into the systemic circulation from the posterior pituitary in response to tactile stimulation of the reproductive tract, particularly the cervix. This is known as the Ferguson reflex and is outlined in Figure 23.3. There is also a local (paracrine) release of oxytocin from the fetal membranes, decidua and placenta (Reis et al 2001). There is little evidence that oxytocin initiates or is the primary uterotonin of labour, and it can only be used clinically to induce labour if the cervix is ripe (Tenore 2003). Plasma oxytocin levels do not increase physiologically until the second stage of labour, although local concentrations may increase before this. The importance of oxytocin for labour is demonstrated by the increase of oxytocin receptors in the decidua during pregnancy, reaching a 300-fold increase by term (Zeeman, cited in Blackburn 2007; Takemura, cited in Reis et al 2001). Oxytocin binds to these decidual receptors, stimulates the release of prostaglandins from the decidua and stimulates uterine pacemakers.
Oestrogen
Oestrogen has a primary role in initiating many changes that are essential for labour, and levels begin to rise at about 34 weeks gestation. Oestrogen increases the sensitivity of the myometrial oxytocin receptors during pregnancy (Blackburn 2007) and thus facilitates myometrial contractility. Oestrogen promotes the formation of CAPs and prostaglandin synthesis via increases in phospholipase A2 release from lysosomes (Johnson 2007). The synthesis of oestriol (the active oestrogen in pregnancy) relies on the fetal adrenal precursor DHEAS as outlined earlier (see Fig 23.1). Therefore, maternal serum oestriol levels are an indicator of fetal hypothalamic–pituitary–adrenal maturity and elevated levels of oestriol are associated with the onset of labour (Norwitz et al 1999).
Progesterone
Progesterone is also a pregnancy hormone and, like oestrogen, is produced in the placenta. However, in contrast to oestrogen, it is not reliant on fetal precursors for synthesis and its role is in suppressing uterine excitement. Progesterone does this by promoting the uptake of intracellular calcium into the sarcoplasmic reticulum of the myometrium, and works with nitric oxide to inhibit the production of CAPs (Challis 2001). Progesterone also has a role in decreasing oxytocin binding during pregnancy, and it is understood that removal of this effect at the onset of labour could facilitate myometrial contractions (Thornton et al 1999). Maternal plasma progesterone does not decrease at parturition, as it does in some animals (Norwitz et al 1999), but there may be local decreases in progesterone activity through increased progesterone-binding protein. The decreased availability of progesterone and increased synthesis of oestrogen produce an increased oestrogen-to-progesterone ratio, allowing the uterotonic effects of oestrogen to dominate, enabling contraction of uterine muscle.
Relaxin
Relaxin, like progesterone, supports quiescence of the uterus. Relaxin is produced by the myometrium, decidua and placenta. Its levels are highest in the first trimester, but are measurable throughout pregnancy. Relaxin has a role in enhancing cervical ripening; although exogenous application of relaxin promotes cervical ripening, the implications of clinical use of the hormone are not clear (Kelly et al 2001).
Nitric oxide
Nitric oxide (NO) is important in maintaining myometrial quiescence (Longo et al 2003). It is produced by the decidua, fetal membranes, and fetal and placental vascular epithelium. Nitric oxide is believed to interact with progesterone to inhibit the production of CAPs (Challis 2001). Levels of NO in the myometrium decrease near term but increase in the cervix. Nitric oxide production is dependent on the enzyme nitric oxide synthetase (NOS), which has three forms. There is an increase in the expression of one form, iNOS, in the cervix prior to the onset of labour, suggesting that NO contributes to cervical ripening (Ledingham et al 2000).
Summary: the hormonal contribution to labour
• Systemic plasma levels of hormones are not representative of local changes that may initiate and maintain labour.
• During pregnancy, hormones such as progesterone, relaxin and nitric oxide maintain the uterus in a quiescent state.
• Prior to labour, changes in the oestrogen-to-progesterone ratio facilitate activation of the uterine muscle, and ripening of the cervix occurs under the influence of prostaglandins, relaxin and nitric oxide.
• Following activation of myometrial cells and cervical ripening in the uterus, the myometrium responds to the uterotonins, oxytocin and prostaglandins.
• Initiation of labour probably involves both fetal and maternal influences.
FIRST STAGE OF LABOUR
During the first stage of labour, myometrial contractions lead to effacement and dilation of the cervix. The first stage is often divided into two phases, latent and active. In the latent phase, uterine contractions occur but cervical dilation is slow. The timeframe for this phase varies considerably (Enkin et al 2000). In the active phase, strong, effective contractions lead to cervical dilation. Cervical dilation of 3 cm is sometimes regarded as an indicator of active labour (Johnson 2007).
Uterine contractions during the first stage
Uterine muscle cells undergo some unique changes. For example, pacemakers, located in each cornu of the uterus, initiate contractions under the influence of local hormones. Gap junctions between the myometrial cells facilitate the rapid spread of electrical activity downward over the muscle cells, facilitating almost synchronous contraction of the myometrium so that maximum tension is generated in all myometrial cells at the same time. However, as expected, the strength of contraction is greater in the fundus and less in the lower uterine segment, due to the relatively low muscle and high connective tissue content in the lower region of the uterus. Along with synchronous contraction, smooth muscle can also maintain tone between contractions, a characteristic called retraction. Due to retraction, the smooth muscle cells of the upper segment never fully relax and thus each contraction causes the muscle cells to become progressively shorter and fatter. Retraction of the upper segment enables shortening of the lower uterine segment so that the cervix becomes continuous with the lower uterine segment—that is, effacement. The process is illustrated in Figures 23.4 and 23.5.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree