Endocrine changes in pregnancy
Steroid hormones
Human chorionic gonadotrophin
Human placental lactogen
Relaxin
Adrenal and pituitary hormones
Thyroid hormones
The reproductive system
The blood vessels
The uterus
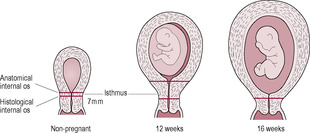
Fig. 11.2
The cervix
The vagina
The breasts
The cardiovascular system
Blood volume
Cardiac output
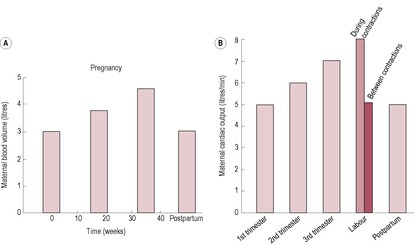
Fig. 11.5
Heart
Arteriovenous (AV) difference
Blood pressure
Distribution of blood flow
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Physiological adaptation to pregnancy
Zara is now in the third trimester of her pregnancy. She informs her midwife that she is not sleeping well, and James prefers to sleep in the spare room as Zara’s loud snoring and frequent waking disturbs him. When Zara does sleep, she experiences vivid dreams that cause her to wake suddenly. Her sleeping is not helped by the fact she often wakes up feeling hungry and thirsty and is having to make increasingly frequent visits to the toilet. Zara has now stopped working and finds that the only way she can cope is to a have a rest in the afternoon. She often falls asleep sitting in front of the television for 3–4h and says this afternoon sleep is less disturbed than the night time.
• What physiological changes could account for these changes in Zara’s sleep pattern and what reasons would you give to reassure Zara that this is normal?
• Why do you think Zara is able to sleep more peacefully in the afternoon?
• Are there any advantages in these behavioural changes and might they be preparing Zara to care for her newborn baby?
The physiological changes of pregnancy are controlled by an alteration in hormone secretion. The trophoblastic cells produce human chorionic gonadotrophin (hCG), which stimulates secretion from the corpus luteum, increasing ovarian steroid hormone production. As the placenta develops, it also produces oestrogen and progesterone. However, placental endocrine function is much broader as the placenta synthesizes a range of hormones and releasing factors that are similar to those originating from the hypothalamus and other maternal endocrine organs (see Chapter 8). Placental products may reach both the maternal and fetal circulation, thus regulating maternal physiology and fetal development.
Steroidogenesis depends on interaction and cooperation between the mother, placenta and fetus. The mother is the source of precursors for placental progesterone production and the fetus is the source of precursors for placental oestrogen production. Placental progesterone is used for fetal synthesis of testosterone, corticosteroids and mineralocorticoids. Progesterone is known as the hormone of pregnancy; it stimulates respiration, relaxes smooth muscle (of blood vessels, uterus and gut), increases body temperature, increases sodium and chloride excretion, and may act as an immunosuppressant in the placenta (Picciano, 2003). It is suggested that the repeated miscarriages might be associated with decreased progesterone levels due to stress (Arck et al., 2007). Stress affects the hypothalamic–pituitary–adrenal (HPA) axis and increases levels of the classical stress mediators, corticotrophin-releasing hormone (CRH) and glucocorticoids. CRH can inhibit GnRH and glucocorticoids can suppress pituitary luteinizing hormone (LH) secretion and thus affect steroid hormone production.
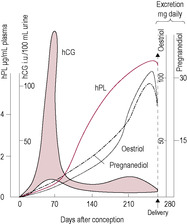
Progesterone levels increase gradually at first (Fig. 11.1). There is little change in progesterone concentration between the 5th and 10th week, but after the 10th week the levels increase more markedly as the placenta becomes the main site of steroid hormone synthesis. By the end of the first trimester, levels of progesterone are 50% higher than luteal levels and by term the levels have increased threefold. The syncytiotrophoblast uses maternal cholesterol as a substrate for progesterone synthesis. Placental production of progesterone is adequate by 5–6 weeks. A primate pregnancy can survive ovariectomy (oophorectomy, removal of ovaries), although the corpus luteum is essential in other mammalian pregnancies (Johnson and Everitt, 2000). Human corpus luteal production of 17α-hydroxyprogesterone decreases from 6 to 9 weeks as placental production increases. Measurement of the different progesterone metabolites was commonly used to assess placental function.
The primary oestrogen of pregnancy is oestriol. Early in pregnancy, oestrone and oestradiol levels increase but oestriol levels do not begin to rise until the 9th week when the fetal adrenal glands begin to synthesize the precursor dehydroepiandrosterone sulphate (DHEAS) from placental pregnenolone; DHEAS is the substrate for placental production of oestriol (see Chapter 3). Maternal and placental steroids are conjugated in the fetal liver and adrenal glands into water soluble, and thus biologically inactive, forms (so the fetus is protected from the effect of the steroids’ precursors). As the 16-hydroxyl precursor originates only from the fetal liver, production of oestriol indicates fetal well-being. In ‘at risk’ pregnancies, decreased oestriol may indicate fetal distress and the need to induce premature delivery, although as an index of placental function and fetal well-being it has largely been replaced by Doppler investigation and biophysical profiling. Oestriol measurement is part of the Bart’s (triple) test for Down’s syndrome (see Chapter 7). Oestrone and oestriol levels increase about 100 times and oestradiol levels about 1000 times during the course of the pregnancy (Blackburn, 2007). The oestrogens promote the growth of the endometrium and breasts, enhance myometrial activity, increase sensitivity to carbon dioxide, increase pituitary prolactin secretion, promote myometrial vasodilation, stimulate fluid retention, alter the composition of connective tissue and increase the sensitivity of the uterus to progesterone in late pregnancy.
The main function of hCG is to maintain production of steroid hormones from the corpus luteum in early pregnancy until the placenta can take over. hCG has a very similar structure to that of LH – as well as follicle-stimulating hormone (FSH) and thyroid-stimulating hormone (TSH) – and acts on the LH receptors, prolonging the life of the corpus luteum. hCG is produced initially by the outer cells of the blastocyst which are the cells that differentiate into trophoblast cells and subsequently into the placenta. The syncytiotrophoblast, which evolves from the trophoblast (see Chapter 8), continues to produce hCG. It is secreted, and can be detected before implantation, in vaginal secretions and in the maternal circulation (see Fig. 11.1). As the lacunae begin to be formed by the invading syncytiotrophoblast, the hCG diffuses into the maternal blood and significant levels can be detected. Measurable urine values are present 2 weeks after fertilization; home pregnancy tests are very sensitive and specific. The presence of hCG confirms successful fertilization as, apart from very rare production by certain gut tumours, it is not produced by other tissues (Iles and Chard, 1991). hCG is produced in large amounts by hydatidiform moles (see Chapter 8); following evacuation of a molar pregnancy, urinalysis for the presence of hCG is continued for 2 years to exclude the development of choriocarcinoma.
Production of hCG is maximal at 8–10 weeks and then falls to a low plateau level that is maintained throughout the pregnancy. hCG levels, therefore, reflect the placental transformation from an organ of invasion to one of transfer. Persistently low levels of hCG are associated with abnormal placental development or ectopic pregnancy. If hCG is given to non-pregnant women, the corpus luteum is maintained and progesterone secretion rises. Alternatively, antibodies to hCG given to a pregnant woman cause the corpus luteum to regress. hCG, rather than LH, is used to induce ovulation in fertility treatment. By 4–5 weeks, the placenta and fetus are synthesizing significant amounts of steroid hormones and can assume endocrine control of the pregnancy. hCG has thyroid-hormone-stimulating properties, affecting appetite and fat deposition, and also affects thirst, release of antidiuretic hormone (ADH) and other osmoregulatory changes (Davison et al., 1990). It also promotes myometrial growth and inhibits myometrial contractility (Kornyei, Lei and Rao, 1993). The effects of hCG are summarized in Box 11.1.
Box 11.1
• Luteotrophic effect on corpus luteum that maintains synthesis and secretion of oestrogen and progesterone
• Simulates placental progesterone production
• Possesses thyrotrophic activity
• May be responsible for nausea and vomiting
• Stimulates maternal thyroid gland; increases appetite and fat deposition
• Increases sensitivity to glucose
• Decreases osmotic threshold for thirst and release of ADH
• Suppresses maternal lymphocyte response thus preventing rejection of the placenta
• Promotes myometrial growth
• Inhibits myometrial contractility
• Modulates trophoblastic invasion
• Affects fetal nervous tissue development
• Affects male sexual differentiation and stimulates fetal testes to produce testosterone
• Stimulates fetal adrenal glands to increase production of corticosteroids
As hCG levels fall, there is increased secretion of human placental lactogen (hPL). The levels of hPL increase in parallel with the size of the placenta and correlate well with fetal and placental weight. hPL has a similar structure and properties to growth hormone and prolactin; it is a single polypeptide chain and is lactogenic and stimulates growth of both maternal and fetal tissues. hPL appears to protect the fetus from rejection, and low levels of hPL are associated with pregnancy failure and spontaneous abortion. hPL is antagonistic to insulin, resulting in increased maternal metabolism and utilization of fat as an energy substrate. This diabetogenic effect of pregnancy reduces glucose uptake by maternal cells, thus making more available for fetal use (see below). hPL is also called human chorionic somatomammatrophin.
Relaxin is produced by the corpus luteum, and to a lesser extent by the myometrium and placenta. Levels of relaxin are highest in the first trimester. Relaxin has a role in the softening of elastic ligaments of pelvic bones and has been used clinically to enhance cervical ripening during induction of labour (see Chapter 13). Relaxin acts with progesterone to maintain uterine quiescence; it may also suppress oxytocin release and affect gap junction permeability. The softening and relaxation of the pelvic ligaments allow mobilization and growth of the uterus into the abdomen. Sometimes women experience low-back pain in pregnancy, which is associated with the stretching of these ligaments. For some women, this results in the pelvic joints becoming unstable and in severe cases results in symphysis pubis dysfunction (SPD), with severe pain on walking. Relaxin is also thought to be involved in endometrial differentiation during embryo implantation, wound healing and, possibly, tumour growth and progression (Ivell and Einspanier, 2002).
The adrenal gland increases in both size and activity during pregnancy. Oestrogen stimulates adrenal cortisol production by inhibiting the metabolism of cortisol and increasing the synthesis of cortisol-binding protein (transcortin). Progesterone increases tissue resistance to cortisol by competing at the receptor level and binding to the cortisol-binding protein; this also results in an increase in cortisol production. CRH from the hypothalamus affects the release of adrenocorticotrophin (ACTH), melanocyte-stimulating hormone (MSH) and β-endorphin from the anterior pituitary gland. ACTH stimulates the adrenal gland production of cortisol. Cortisol levels increase in response to stress, including increased cardiac output and decreased fasting glucose levels in the second trimester of pregnancy. Both CRH and ACTH are also produced by the placenta as well as by the maternal hypothalamic–pituitary axis; the placental hormones are subject to different feedback control mechanisms and may be important in initiating labour (see Chapter 13).
The increase in circulating levels of cortisol has a positive effect on certain conditions, such as rheumatoid arthritis and eczema. This observation led to the clinical use of exogenous cortisol as a treatment for these conditions. Both progesterone and oestrogen act synergistically to increase aldosterone production. Adrenal synthesis of androgens, oestrogen, progesterone and cholesterol increases in pregnancy.
The pituitary gland enlarges markedly during pregnancy. Much of the increase is due to increased number and activity of cells of the anterior pituitary gland. The gonadotrophs decrease in number as the raised oestrogen concentration inhibits release of FSH and LH, which are barely detectable for most of the pregnancy. However, under the influence of progesterone and oestrogen, the prolactin-secreting cells increase from 10% of the cell population to 50%. Prolactin levels increase progressively through the pregnancy to values 20 times higher than the prepregnant level. Production of ACTH increases, resulting in increased adrenal activity. MSH synthesis also increases so hyperpigmentation may occur (Elling and Powell, 1997). Pregnant women frequently observe that they tan more deeply or develop irregular pigmented patches. Towards the second half of pregnancy, oxytocin production from the posterior pituitary increases.
The hypothalamic–pituitary–thyroid axis undergoes marked changes in pregnancy. Oestrogen, hCG and altered hepatic and renal function together act to change the levels of tri-iodothyronine (T3), thyroxine (T4) and thyroid-binding globulin (TBG); these changes are important to support the altered metabolism of pregnancy. Oestrogen stimulates hepatic synthesis of TBG by 50–100 times resulting in increased total amounts of T3 and T4, although free concentrations remain within normal physiological limits. hCG has mild TSH activity so it stimulates both the production of T4 and the deiodination of T4 to T3 in the peripheral tissues. The high concentrations of hCG in the first trimester and the consequent increased secretion of thyroid hormones results in TSH release from the pituitary gland being inhibited. This means that maternal circulating concentrations of free T3 and free T4 peak at the end of the first trimester (de Escobar et al., 2008) requiring an increased availability of iodine. If iodine is limiting, the maternal thyroid exhibits an autoregulatory response, increasing synthesis of T3 (which requires 3 iodine atoms per molecule) at the expense of T4 production. Thus there is no change in TSH but there will be less T4 transported across the placenta to the fetus which could compromise neurodevelopment. Thyroid hormone receptors can be demonstrated in the fetal brain early in development (Bernal and Pekonen, 1984) suggesting early brain development requires maternal thyroid hormone input (later in gestation, both the maternal and fetal thyroid glands provide T4). It should be noted that production of sufficient thyroid hormone requires a doubling of iodine intake compared to pregnancy requirement (which may mean potassium iodide supplements should be recommended – see Chapter 12).
Pregnancy mimics hyperthyroidism in a number of respects, for instance by increasing body temperature, and stimulating appetite and feelings of fatigue. In most pregnant women, the thyroid gland enlarges because thyroid activity increases and renal iodine loss is increased. Ancient Egyptians used the observation of pregnancy-induced goitre (thyroid gland hypertrophy) as confirmation of pregnancy (Glinoer and Lemone, 1992). The thyroid gland hypertrophies as it attempts to increase uptake of iodine for hormone synthesis. Nowadays, maternal goitre is rare in pregnancy partly because of better diets and iodine supplementation of table salt, but subclinical iodine deficiency can compromise fetal brain and central nervous system development resulting in neurological anomalies, reduced cephalic size (microcephaly) and reduced intelligence quotient (IQ). Many women are at the threshold of iodine deficiency and the demands of pregnancy can compromise them further, adding support for routine iodine supplementation in pregnancy (Perez-Lopez, 2007). Basal metabolic rate increases by 20–25% from the 4th month of pregnancy but much of the increase is related to the increased surface area of the mother and the increased work she has to do maintaining maternal and fetal tissue requirements. Nausea and vomiting have been linked not only to the changes in hCG (see above) but also directly to the rise in free T4.
Hypothyroidism is common in women of reproductive age and in pregnancy can be associated with adverse maternal and neonatal outcomes including increased risk of pregnancy-induced hypertension, miscarriage, still-birth, postpartum haemorrhage, congenital malformation and fetal distress (Idris et al., 2005). Women with pre-existing thyroid deficiencies require close monitoring of thyroid function during pregnancy and often require increased doses of thyroxine due the altered metabolic demands of pregnancy.
The vasculature of the uterus undergoes a number of remarkable and unique changes during pregnancy. Uterine blood flow increases: the vessel diameter of the spiral arteries increases and vascular resistance falls (see Chapter 8). These essential changes accommodate the increased blood flow to the placenta, which is maintained under conditions of low blood pressure. The coursing of blood through the enlarged tortuous arteries produces a uterine ‘souffle’, which may be heard through a stethoscope or with a sonicaid. The enhanced blood supply to the uterus and the concomitant establishment of the maternal circulation to the placenta effectively diverts blood away from the legs (Burton et al., 2009).
The uterus increases in all dimensions and also changes shape (see Chapters 2 and 13) during pregnancy. Uterine hyperplasia begins after implantation and is driven by oestrogen and growth factors (it occurs if the embryo is implanted in an extrauterine site; Blackburn, 2007). Early growth results in thickening of the uterine wall. The endometrium thickens into the decidua. The three layers of the myometrium become clearly defined as the uterine muscle undergoes initial hyperplasia (development of new fibres) and subsequent hypertrophy (increase in length and thickness of existing muscle fibres). Later uterine growth is mostly hypertrophy and hyperplasia of the myocytes and remodelling of connective tissue which is driven by distension as the fetus enlarges. The muscle fibres increase in length and width as the timing and speed of the myometrial action potentials change and the muscle cells increase their content of actin and myosin, gap junctions, sarcoplasmic reticulum and mitochondria.
In early pregnancy, the uterine isthmus increases from about 7 to 25 mm (Fig. 11.2). From 32 to 34 weeks, the isthmus forms the lower uterine segment (LUS). As effacement commences (at approximately 36 weeks), the external os is incorporated into the LUS (see Chapter 13). The blastocyst usually implants in the fundus (upper part) of the uterus. By 12 weeks, the fetus fills the uterine cavity and the fundus can just be palpated (felt) at the pelvic brim. By 20 weeks the fundus reaches the maternal umbilicus and by 8 months it reaches the sternum. As the uterus expands during pregnancy, it loses its anteverted and anteflexed configuration and becomes erect, tilting and then rotating to the right under the pressure of the descending colon. The uterus changes from its non-pregnant pear-shape and becomes spherical and then cylindrical. Abdominal measurement using the symphysis pubis as a reference point is often used to assess uterine size and fetal growth as pregnancy progresses.
(Reproduced with permission from Miller and Hanretty, 1998.)
Uterine quiescence is mediated by progesterone, relaxin, nitric oxide (NO) and prostacyclin (also known as prostaglandin PGI2). The uterus is never completely quiescent and exhibits low-frequency activity throughout the pregnancy (as it does in the non-pregnant state). Braxton Hicks contractions are painless contractions that are measurable from the first trimester of the pregnancy. These contractions do not dilate the cervix but assist in the circulation of blood to the placenta. The contractions are usually irregular and weak, unsynchronized and multifocal in origin. The contractions of the circular muscles are less than those of the longitudinal ones (Blackburn, 2007). The uterine ligaments soften and thicken under the influence of oestrogen, resulting in increased mobility and capacity of the pelvis.
The cervix increases in mass and width during the pregnancy. Oestrogen increases the blood supply to the cervix resulting in a lilac coloration and softer tissue texture; the water content of the cervix also changes. The collagen fibre bundles become less tightly bound (see Chapter 13). The cervical mucosa proliferates and the glands become more complex and secrete thickened mucus, which forms a plug or operculum protecting the cervix from ascending infection. The plug is held laterally by projections of thickened mucus in the mouths of the mucus-secreting glands. It is this plug that is released as ‘the show’ at the onset of labour when the cervix starts to be drawn up to form the LUS.
Blood flow to the vagina increases likewise, resulting in softer vaginal tissue which is more distensible. The lilac coloration of the vagina and cervix was traditionally recognized as being an indicator of pregnancy (described as Jacquemier’s sign). The increased blood flow means that the pulsating of the uterine arteries can be felt through the lateral fornices (Osiander’s sign). The increased vascularization of the vagina can result in increased sensitivity and sexual arousal. Venous engorgement results in increased vascular transudation, which together with the increased cervical mucus production results in an increased vaginal discharge. The vaginal discharge (leucorrhoea) has a low pH (because of the effect of raised oestrogen levels on the vaginal flora) and is thick and white with an inoffensive odour. Oestrogen also stimulates the vaginal epithelial cell division so the cells acquire a distinctly boat-shaped appearance (which should not be mistaken for carcinoma cells). Early in pregnancy, the hypertrophied corpus luteum, which is about 3–5 cm long, distends from the ovarian surface; this may be palpated in some women or visualized during endoscopic examination in women undergoing egg retrieval for IVF (in vitro fertilization).
In early pregnancy, vascularization of the breasts increases. This tends to result in a marbled appearance of the skin owing to the marked dilation of the superficial veins. The breasts, specially the nipple areas, may feel sensitive and tingle because of the engorgement of blood. (Changes to the breast in pregnancy are described in more detail in Chapter 16.) Pregnancy following diagnosis and treatment for breast cancer is becoming more common with earlier diagnosis and delayed childbirth. Although concerns have been raised about the possible promotional effects of raised oestrogen levels during pregnancy on residual metastatic disease, the majority of studies indicate no adverse effect on the outcome of pregnancy or on survival (de Bree et al., 2010).
The signs of pregnancy are summarized in Box 11.2.
Box 11.2
• Amenorrhoea
• Softening of vagina and cervix
• Increased blood flow to vagina and cervix causing lilac coloration (Jacquemier’s sign)
• Pulsating of uterine arteries (Osiander’s sign)
• Tingling and sensitive breasts with dilated superficial veins marbling surface
• Nausea and vomiting, possible changes in taste
• Increased frequency of urination as uterus compresses bladder
• Increased pigmentation of skin
• Bleeding gums
• Tiredness
• Increased appetite and thirst
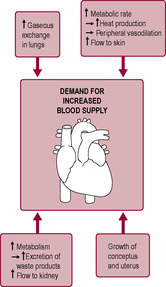
The most notable physiological changes occur in the cardiovascular system in preparation for the increased demands of maternal and fetal tissues (Fig. 11.3). These changes are caused both indirectly by hormones (oestrogen, progesterone, prostaglandins and other vasoactive substances) and directly by mechanical effects and as a result of the increased load on the system. Marked haemodynamic adjustments take place in pregnancy; maternal blood volume and cardiac output quickly increase but paradoxically blood pressure falls because of the marked reduction in systemic vascular resistance and reduced blood viscosity due to haemodilution. Heart disease affects less than 1% of pregnancies and causes 10 deaths per million in England and Wales, but symptoms of heart disease (such as breathlessness, palpitations, fainting and oedema) are present in over 90% of pregnant women (de Swiet, 1998a). Superimposed on a pre-existing cardiac disease state, pregnancy may be dangerous and even potentially fatal. Measurement of cardiovascular system parameters is technically difficult and notoriously variable. Measurements obviously have to be indirect and are very sensitive to changes such as emotion, exertion and posture. In the research literature, there are many inconsistencies, some of which reflect differences in standardization of conditions (de Swiet, 1998a). In the last couple of decades there has been an increased incidence of myocardial infarction (MI) in pregnancy which reflects the increasing proposition of older women having babies (the risk of MI is 30-times higher in women over 40 years compared to women under 20 years; Curry et al., 2009) and increased obesity and pre-existing diabetes (Ward et al., 2007).
Total blood volume increases by 30–50%, more in multiple pregnancies (Brown and Gallery, 1994), resulting in a fall in plasma osmolality. The rise in blood volume correlates well with birth weight and, as it begins early in pregnancy, the mechanism of these early changes in the cardiovascular system is thought to be hormonally driven. It has been disputed whether the increase in volume (sodium and fluid retention) precedes the increased vascular space (‘overfill’ hypothesis) or whether the changes are stimulated by relative hypovolaemia (increased vascular capacity), known as the ‘underfill’ hypothesis (Schrier, 1992). The arguments for the ‘underfill’ hypothesis are supported by the observation that blood pressure falls before plasma volume expands (Duvekot et al., 1993). The consensus is that both mechanisms are important but the initial change is probably the decreased systemic resistance (Ward et al., 2007). Often in early pregnancy women feel faint, suggesting that the physiological compensation of the underfill has yet to occur.
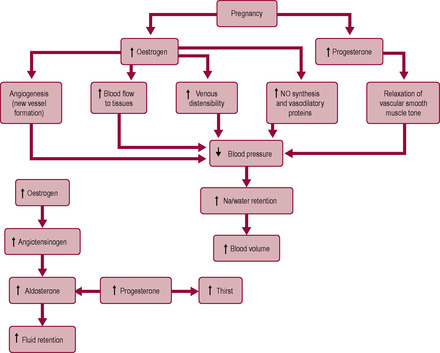
Oestrogen stimulates angiogenesis (formation of new blood vessels and vascular beds) and increases the blood flow to the tissues. Oestrogen affects the distribution of collagen in the tunica media of the large vessel walls, increasing venous distensibility. Oestrogen also stimulates endothelium-dependent vasodilatation, by increasing synthesis of NO (a potent vasodilator) and vasodilatory prostaglandins and inhibiting the release of endothelin-I (a vasoconstrictor). Production of both prostacyclin and NO increases in pregnancy (Morris et al., 1996). These signals affect placental blood flow, particularly remodelling of the spiral arteries (see Chapter 8).
Progesterone relaxes vascular smooth muscle causing systemic vasodilatation and decreased peripheral resistance, probably via vasoactive prostaglandins and enhanced nitric oxide (NO) production. The syncytiotrophoblast is an essential site of NO production which is important in maintaining vasodilation of the uterine blood vessels and ensuring a high flow and low resistance blood supply to the uteroplacental bed and fetus. The effect of the vasodilation is that the circulatory system increases its capacity and is relatively underfilled. One of the early effects of this is decreased glomerular arteriolar resistance which results in increased glomerular filtration rate (GFR) and renal blood flow (Ward et al., 2007). Relaxin production is stimulated by hCG and is also involved in renal vasodilation (Conrad et al., 2005). The decreased vascular tone in the blood supply to the kidneys causes renal compensatory mechanisms to increase plasma volume and cardiac output. In addition, both progesterone and oestrogen increase water retention by affecting the renin–angiotensin system (RAS) and oestrogen increases hepatic angiotensinogen production. This results in a rise in angiotensin II, which increases renal fluid resorption and stimulates the production of aldosterone. All components of the RAS increase in pregnancy but there is decreased sensitivity to vasoconstrictors such as angiotensin II and noradrenaline during pregnancy (so blood pressure does not rise). Renin is produced by the uterus, placenta and fetus, as well as the kidney. Levels of renin are two- to threefold higher than before pregnancy. These changes in the RAS may mediate the oestrogen-stimulated angiogenesis and increased cell growth and division. Relaxin increases production of ADH and oxytocin and modulates responses to angiotensin II. The increased ADH promotes water retention and thirst; the raised oxytocin promotes vasodilation and sodium excretion partly by increasing cardiac atrial natriuretic peptide production (Brunton et al., 2008).
Progesterone stimulates a 10-fold increase in the amount of circulating aldosterone. Progesterone is antagonistic to aldosterone but some progesterone is converted to deoxycorticosterone, which has mild aldosterone-like properties. Progesterone augments its effects on the circulatory volume by resetting the thirst centres in the hypothalamus and increasing thirst. Progesterone also lowers the sodium threshold for the RAS and blocks the vasopressive activity of angiotensin II in pregnancy (Blackburn, 2007). The net result of the changes in oestrogen and progesterone is an increase in vascular resistance followed by increased sodium and water retention and expansion of the circulating volume (Fig. 11.4).
Blood volume and cardiac output increase in parallel (Fig. 11.5). Cardiac output increases by 30–50%, an average increase of 1.5 L/min from 4.5 to 6 L/min. Cardiac output rises quickly in the first trimester and is maintained throughout the pregnancy. The increase in cardiac output is greater in multiple pregnancies. Cardiac output is affected by posture: when the pregnant woman lies supine, her uterus impedes venous return from the inferior vena cava resulting in an apparent decrease in cardiac output. The measured drop in cardiac output in the third trimester, observed by a number of researchers, was most probably the result of measurements being made with the woman lying supine (Mabie et al., 1994). In labour, cardiac output increases by about 2.0 L/min.
(Reproduced with permission from Chamberlain et al., 1991. (B) After Whitfield, 1986.)
Cardiac output is the result of two variables: heart rate and stroke volume (see Chapter 1). In pregnancy, both heart rate and stroke volume increase. Heart rate increases soon after implantation, by about 20% (an average of 15 beats more per minute) from about 70 to 85 beats/min. Stroke volume typically increases by about 10% from 64 to 71 mL. Steroid hormones and prolactin may affect the myocardium directly. Oestrogen stimulates an increased accumulation of components of the myocardial cells and increases contractility (Duvekot and Peeters, 1998). Heart rate is usually measured by the palpation of peripheral pulses and the increase in stroke volume means the pulse is easy to palpate in a pregnant woman. Heart rate is affected by many things; tachycardia (fast pulse) may be caused by excitement, stress, fear, medication, illegal use of drugs, etc. and so, in isolation, can be a poor indicator of physical problems such as sepsis and haemorrhage so needs to be considered in conjunction with other abnormal observations such as raised blood pressure, temperature, or respiratory rate.
Bradycardia is an abnormally slow heartbeat and is rare in pregnant women but can indicate heart block, raised intracranial pressure, medication and use of illegal drugs.
The early changes relating to the heart occur early in the pregnancy and are caused by hormonal changes. Later, the heart is displaced upwards by elevation of the diaphragm and is rotated forward so the electrocardiogram (ECG) changes and the location of the apex beat is directed forward to the anterior chest wall. The heart increases in size by an average of 70–80 mL (about 12%). This increase is due to increased filling and oestrogen-stimulated cardiac muscle hypertrophy (an increase in the size of pre-existing cardiomyocytes). The remodelling of the heart that occurs in pregnancy in response to increased blood volume and workload is an adaptive response analogous to the ventricular hypertrophy of an athlete’s heart in continuous training. However, heart hypertrophy can lead to cardiac disturbances such as cardiac arrhythmias (Eghbali et al., 2006). The pregnant woman’s heart is thus dilated and has increased contractility. Increased blood volume results in an increase in venous return and therefore increased atrial size. The heart sounds change because the mitral valve closes marginally before the closure of the tricuspid valve; thus the first heart sound is louder with an exaggerated split. Many pregnant women (92–95%) develop innocent (non-significant) systolic murmurs in pregnancy. The increased blood flow through the mammary blood vessels may be perceived as a possible heart murmur; this is more common in lactation. The net result of increased contractility, increased venous return, cardiac hypertrophy, decreased peripheral resistance and increased heart rate is increased cardiac output. Women with known pre-existing cardiac disease must be carefully monitored during pregnancy as they may not have the physiological reserve to cope with this increased demand on the heart (see Case Study 11.1). Other conditions may also pose serious risk in pregnancy relating to increased cardiac output such as Marfan syndrome (see Box 11.3).
Moira is a 23-year-old primigravida who, at the age of 19 underwent a heart and lung transplant as a result of cystic fibrosis. Moira and her partner had been well informed of the risks associated with a pregnancy, had planned not to have any children and so this pregnancy was unexpected.
• How should Moira’s care be managed in relation to her transplant status and her pregnancy and what is the role of the midwife in this complex case?
• What are the possible complications and risks in this case and what would the midwife need to know and do to ensure early recognition, referral and intervention is optimized?
Box 11.3
Marfan syndrome is a disorder of connective tissue and as a result has a high incidence of aortic aneurism due to the inherent weakness of the artery wall. Incidence of the disease is about 2 per 10000 births and affects both men and women equally. As cardiac output is increased in pregnancy, the risk of aortic aneurism occurring is greatly increased and so the pregnant woman should have regular cardiac ultrasounds to optimize early detection of this potentially life threatening situation. If aortic aneurism occurs, emergency surgical intervention is required which involves the weakness of the aortic wall being strengthened by synthetic graft material.
Increased cardiac output exceeds increased oxygen consumption (especially early in pregnancy when cardiac output increases considerably and oxygen consumption is relatively low) so more oxygen is returned to the heart from venous circulation compared with prepregnant values and the AV difference is smaller. The AV difference is 34 mL in mid-pregnancy rising towards term but is always less than the non-pregnant values of about 45 mL (de Swiet, 1998a). The higher return of oxygen to the heart suggests that the commonly measured decrease in haemoglobin concentration is not physiologically inadequate and the relatively small increase in total haemoglobin (oxygen-carrying capacity) is more than sufficient to compensate for increased oxygen requirements. This supports the argument that the term ‘physiological anaemia’ is inappropriate (see p. 269). The increased AV difference, especially early in the pregnancy before increased oxygen consumption, means that early fetal development and organogenesis occur in an environment which is adequately oxygenated despite the maternal spiral arteries not connecting with the intervillous spaces in the early part of pregnancy (see Chapter 8).
Normal pregnancy has relatively little effect on arterial blood pressure. Despite increased cardiac output and increased vascular capacitance, there is relatively little change in systolic pressure in pregnancy. However, diastolic blood pressure is lower in the first two trimesters and returns to prepregnant values in the third trimester. Both the development of new vascular beds and the relaxation of peripheral tone by progesterone result in decreased resistance to flow. This is augmented by a change in the profile of prostaglandins produced. The levels of the prostaglandin PGE2 and prostacyclin, which stimulate vasodilation, rise early in pregnancy. NO (nitric oxide; formerly known as endothelium-derived relaxing factor, EDRF) also appears to play an important vasodilatory role (Palmer et al., 1987). The most important stimulator for NO production is shear stress such as that generated from a pulsatile blood flow (Maul et al., 2003). The increased difference between diastolic and systolic blood pressure means that for much of the pregnancy the pulse pressure is increased. Hypotension, particularly in early pregnancy, has been associated with fatigue, headaches and dizziness, which many women experience.
In pregnancy, changes in posture can cause acute haemodynamic changes (Blackburn, 2007). Blood pressure in normotensive women is higher when sitting and falls on lying, especially in the supine position (Box 11.4). Effects on venous pressure are relatively dramatic compared with the effects on arterial pressure. As there are no valves between the return from the femoral veins to the vena cava and heart, venous pressure in the legs is similar to the pressure in the heart so, if a pregnant woman lies in a supine position, the uterus can compress the aorta and, particularly, the thin-walled vena cava and iliac veins. (The aorta is compressed as well but to a lesser degree because it has a much thicker vessel wall.) Return of blood to the heart can also be impeded by the pressure of the fetal head on the iliac veins and by hydrodynamic obstruction due to outflow of blood from the uterine vessels. Most women experience a drop in blood pressure greater than 10% when they lie down; for some of these women this fall is extreme, reaching up to 50%. The effect of assuming the lithotomy position in labour is to decrease cardiac output significantly (Carbonne et al., 1996).
Box 11.4
As high blood pressure in pregnancy is a risk factor for serious maternal and fetal complications, it is important that blood pressure is measured accurately (NICE, 2008). Oscillometric (automated) blood pressure equipment is usually used however this is best used when serial monitoring is required such as in fulminating pre-eclampsia or eclampsia. Routine blood pressure monitoring should be undertaken with manual equipment (NICE, 2008). Important factors that affect the blood pressure reading include correct cuff size and the woman’s position and posture, avoiding recent intake of caffeine or nicotine, taking the average of two readings, etc. The usual clinical definition of hypertension is:
(1) a single diastolic reading of 110 mmHg or above
(2) or a diastolic of 90 mmHg or above (but below 110) on 2 consecutive occasions at least 4h apart
(3) and/or a systolic reading of 160 mmHg or above on 2 consecutive occasions at least 4h apart.
Some women may not show the expected reduction in blood pressure in early pregnancy. This may be due to a degree of pre-existing renal disease or condition causing chronic hypertension but may also be an early indicator of hypertensive disease in pregnancy. Note that pre-eclampsia can be superimposed on chronic hypertension. A rise in blood pressure is often associated but not always present in pre-eclampsia. Other reasons for high blood pressure may be stress and anxiety, acute renal disease such as infection, raised inter-cranial pressure (the significance of this is increased with a slowing pulse). The blood pressure reading needs to be considered in the context of other risk factors. A drop in blood pressure may be caused by haemorrhage (significance is increased with the presence of tachycardia), advanced sepsis (septic shock), and drug induced.
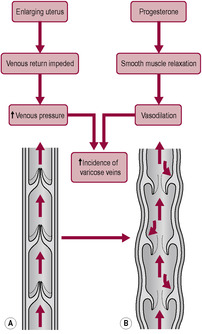
In late pregnancy, most women experience oedema of the lower extremities (see Case study 11.2) owing to the combined effects of progesterone relaxing the vascular tone, the impeding of the venous return by the gravid uterus and gravitational forces. The peripheral circulatory volume is increased by 500–600 mL/limb (de Swiet, 1998a). Oedema is further increased in hypertensive women and tends to increase with increased maternal age. Fluid drunk by the pregnant woman appears as increased leg volume and the expected diuresis is delayed until, she lies down, resulting in increased nocturia. Blood pressure is higher on the side of placental implantation and oedema may also be more marked in the leg on the side of placental implantation (de Swiet, 1998a). The effect of increased venous pressure is to increase the incidence and severity of varicose veins of the legs, vulva and haemorrhoids (Fig. 11.6).
It is the height of summer and Kathy, 38 weeks’ pregnant, informs her midwife she feels fat and sluggish and cannot cope with the hot weather. Kathy’s ankles are visibly swollen.
• Is the midwife right to assume that this is normal?
• What indicators would the midwife be able to use in an assessment to reassure Kathy that all is well?
• What factors may alert the midwife to suspect that all is not well?
Three days later Kathy presents to the midwives’ clinic complaining of breathlessness and chest pain.
• What should the midwife do in response to Cathy’s worsening symptoms?
• What are the possible causes of these symptoms?
The tendency to develop oedema is a also affected by the concentration of plasma proteins (see Box 11.5). The increment in plasma volume is not matched by an increase in plasma protein synthesis so there is decreased plasma colloidal pressure. This, together with the increased venous pressure, means there is an increase in fluid loss from the capillaries. There may also be an increase in capillary permeability (Blackburn, 2007).
Box 11.5
The pressures in the right ventricle, pulmonary arteries and pulmonary capillaries do not change but cardiac output increases. The higher pulmonary blood flow therefore has to be absorbed by decreased pulmonary resistance and dilatation of the pulmonary vascular bed so the volume of pulmonary circulation increases to match the increased cardiac output. Conditions where pulmonary resistance is increased or fixed have a poor maternal prognosis such as Eisenmenger’s syndrome, which has a 30–50% mortality rate. Exercise presents an increased demand on the cardiovascular system (see Box 11.6).
The effects of exercise on the cardiovascular system are summarized in Box 11.6.
Box 11.6
Exercise affects maternal physiology because there is a hormonal response, weight is redistributed and heat is generated. Many women experience very good physical health especially early in pregnancy. However, the question is whether the adaptive response to exercise compromises fetal oxygenation and well-being. The ability to increase cardiac output in response to exercise progressively declines throughout pregnancy. Theoretically, redistribution of weight could affect the venous return and blood could be preferentially circulated to the skeletal muscles and to the skin for heat dissipation. Studies on animals suggest that uterine blood flow can be decreased substantially before fetal oxygen uptake or temperature regulation is compromised. It has been reported that over a third of the female medal winners in the 1956 Russian Olympic team were pregnant and the cardiovascular changes enhanced for their performance (de Swiet, 1998a). In practice, moderate exercise in normal healthy pregnancy is encouraged as maternal and fetal health seem to benefit. However, pregnant women are advised to avoid jumping and jerky movements because of joint instability. Vigorous exercise is not recommended during hot humid weather or if the mother has a fever. It is suggested that heart rate should not exceed 140 beats per minute, strenuous exercise should be done for less than 15 min at a time and a pregnant woman should not allow herself to become breathless. A pregnant woman should stop physical exercise if pain, vaginal bleeding or dizziness is experienced or there are known risk factors.
Oestrogen increases blood flow to all tissues but the distribution of flow is affected by posture. Venous tone is affected by progesterone. The increased venous distensibility results in an increased incidence of varicose veins, venous thrombosis and thromboembolism. The uterus is the central target of the increased circulatory flow during pregnancy but distribution of flow to other organ systems, including kidneys, skin, lungs and breasts, increases as well. It is difficult to distinguish between blood flow to the increasing uterine tissue mass and that going specifically to supply the placenta because the uterine vessels are complex and inaccessible. AV shunts in the uterine vasculature have been identified; these allow a short circuit of the placental site after delivery of the placenta, rather than being important in increased flow during pregnancy. The increased flow to the uterus is about 500 mL/min more than that to the non-pregnant uterus but changes in uterine flow occur relatively late in pregnancy (de Swiet, 1998a). In rare situations of maternal cardiac arrest in late pregnancy, the altered blood flow will affect the ability of external cardiac massage to provide vital organ oxygenation. In such situations, immediate delivery of the infant must be considered either by perimortum caesarean section or an instrumental delivery if in the second stage of labour. Once delivered, the empty uterus will contract down enabling more blood to enter the central circulation improving oxygenation to the vital organs (Lewis, 2007).
Blood flow to the kidneys increases by about 400 mL/min from early pregnancy, facilitating elimination of waste products. Vasodilatory prostaglandins are implicated in the peripheral vasodilation, which is particularly evident in the vessels of the breasts, hands and face. Oestrogen and progesterone depress the normal response to angiotensin II and oestrogen abrogates the vasoconstriction mediated by the sympathetic nervous system. Blood flow to the lungs increases, reflecting the increased circulating blood volume and cardiac output. Distribution of blood to the skin is greatly increased (by about 500 mL/min) expediting heat loss. It is common for pregnant women to complain of being hot. Pregnant women usually have warm hands and feet and often complain that midwives’ hands are cold. This vasodilatory effect is enhanced in smokers. Blood flow to the hands increases about sevenfold giving a very marked increase in skin temperature. The resulting peripheral vasodilation causes the capillaries to dilate and stimulates angiogenesis, and may give rise to the development of vascular spiders and palmar erythema, which is often associated with burning sensations (Henry et al., 2006). The increased blood flow means there is a decreased tendency to arteriolar spasm, and therefore conditions such as Raynaud’s syndrome are abolished.
Get Clinical Tree app for offline access