pH Regulation Through Buffering Systems
Proteins, carbonic acid-bicarbonate, phosphates, and the plasma potassium-hydrogen exchange control the metabolic regulation of the body’s pH, either by working outside the cell, inside the cell, or both inside and outside the cell. The protein and bicarbonate buffering systems are immediately available and the most effective in maintaining the proper body pH.
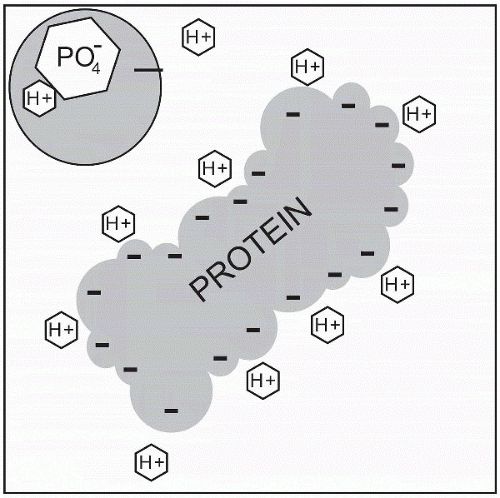
Protein Buffers
Proteins are the most plentiful buffering system, with a negative charge to buffer the hydrogen ion (H+) and release or bind with it. Therefore proteins have the ability to function as an acid or base. Though proteins
exist inside and outside the cell, most are primarily located inside the cell, making this an intracellular buffering mechanism. H+ and CO2 diffuse across the cell membrane to bind with proteins inside the cell, whereas albumin and plasma globulins act as the primary protein buffers in the vascular compartment.
exist inside and outside the cell, most are primarily located inside the cell, making this an intracellular buffering mechanism. H+ and CO2 diffuse across the cell membrane to bind with proteins inside the cell, whereas albumin and plasma globulins act as the primary protein buffers in the vascular compartment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree