Chapter 19 Peritoneal dialysis and home dialysis therapies
Peritoneal dialysis (PD) is an alternative dialytic modality for the patient with chronic kidney disease (CKD). The U.S. Renal Data System (US RDS, 2008) reports that only 8% of the prevalent CKD patient population is on PD. Despite its safety and effectiveness, PD has been in decline since the mid-1990s. PD is primarily a home dialysis therapy for CKD, but it can also be a treatment option for the patient with acute kidney injury in the hospital setting. The range of home therapies for the CKD patient includes PD and home hemodialysis. Home therapy allows patients to remain somewhat independent in their own care and to have greater control over their schedules. Of all home therapies, PD is the most commonly used.
What solutions are used for peritoneal dialysis?
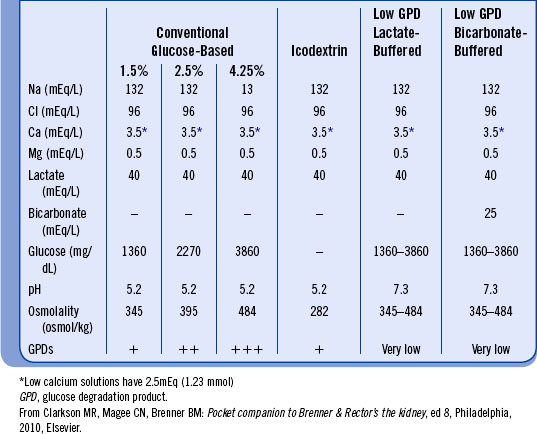
The solutions used for PD should be biocompatible and preserve peritoneal membrane structure and function as much as possible. Conventional solutions use glucose as the osmotic agent and lactate as the buffer base. Commercially available solutions approximate the composition of extracellular body water except for potassium because many patients tend to be hyperkalemic. Potassium may be added (2 to 4 mEq/L) if necessary to correct hypokalemia. Oral potassium supplementation may also be prescribed. Dextrose provides the osmotic gradient between the plasma and the dialysate that leads to fluid and solute removal. The more hypertonic the dialysate is (i.e., 2.5% and 4.25% dextrose), the greater the UF. After a 2-L exchange has been dwelling for 4 hours, an average of 200 mL of ultrafiltrate will be obtained with a 1.5% exchange and an average of 600 to 1000 mL will be obtained with a 4.25% exchange. Table 19-1 highlights some common compositions of peritoneal dialysis solutions.
What is icodextrin?
Newer PD solutions use different osmotic agents for UF and clearance. Icodextrin (Extraneal) is a newer PD solution that differs from standard dialysis solutions in that it does not contain dextrose. In standard PD dialysate, glucose is the osmotic agent. Icodextrin is a starch-derived osmotic agent made from a mixture of glucose polymers (polyglucose). This solution allows for increased fluid removal from the bloodstream during PD as well as reduced net negative UF and increased small solute clearance. Icodextrin is intended to be used for once daily, long dwell exchanges lasting 8 to 16 hours. The dialysate solution should be used for no more than one exchange in a 24-hour period. Icodextrin is contraindicated for those with glycogen storage diseases or an allergy to cornstarch. The most common adverse effect from the use of icodextrin is a skin rash. Sterile peritonitis, hypertension, cold, headache, flulike symptoms, and abdominal pain are other possible side effects.
What are the different forms of automated peritoneal dialysis?
Continuous cycling peritoneal dialysis (CCPD). Three to five exchanges are performed nightly with a full diurnal dwell. The diurnal dwell improves the clearance of middle molecules.
Nocturnal intermittent peritoneal dialysis (NIPD). Three to five exchanges are performed nightly, but there is a minimal or no diurnal dwell. NIPD is indicated in patients who are unable to tolerate a diurnal dwell (e.g., those with hyperpermeability of the peritoneum to dextrose, resulting in absorption of diurnal dwell) and in those with problems exacerbated by increased intraabdominal pressure, including hernias, low back pain, cardiopulmonary compromise, etc.
Intermittent peritoneal dialysis (IPD). Several frequent exchanges are performed three or four times a week, and the peritoneum is left “dry” between treatments. IPD is appropriate for patients with residual renal function or for institutionalized patients. Also, it is used in economically underdeveloped countries because of the financial constraints imposed by daily PD.
Tidal peritoneal dialysis (TPD). An initial volume of dialysate is infused, followed by partial drainage of effluent at the end of each exchange (leaving a constant reserve volume); finally a “tidal” volume of fresh dialysate is infused. TPD is intended to enhance clearance by maintaining continuous contact of dialysate with the peritoneum and maintaining the dialysate/plasma gradient. TPD may improve clearance by 20% but increases costs because of the need for additional dialysate.
TPD may also be used for patients who experience discomfort or “drain pain” at the end of the drain cycle because of the position of the tip of the PD catheter. By always having a reserve of fluid, the tip is allowed to float, thus alleviating discomfort in sensitive individuals.
What kinds of catheters are used for peritoneal dialysis?
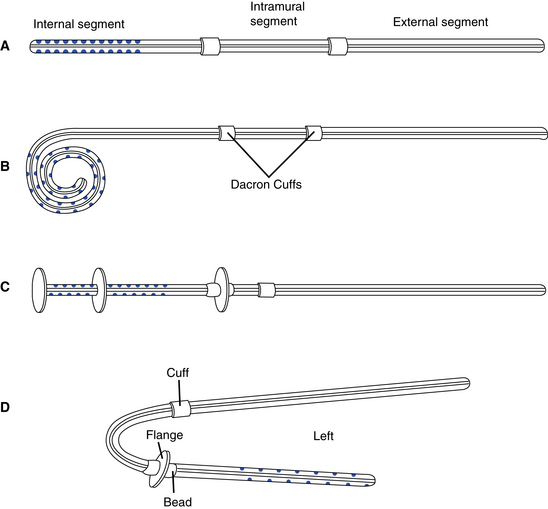
Catheters used for chronic PD are usually placed surgically during a laparotomy or laparoscopically. The exit site should be directed in a downward or lateral direction and be located in the right or left midquadrant area, avoiding the belt line, scars, and skinfolds. Catheters are made of silicone or polyurethane with a radiopaque stripe for x-ray visualization. Catheters may be straight or coiled and have one or two cuffs. Coiled catheters are believed to minimize catheter migration out of the pelvis and have fewer outflow problems than straight catheters. The coiled catheter is also thought to improve patient comfort by keeping the tip of the catheter away from direct contact with the peritoneal membrane.
Cuffs are made of Dacron polyester or velour and provide for tissue ingrowth to stabilize the catheter. Cuffs are also intended to prevent migration of bacteria along the subcutaneous tunnel into the peritoneum. When placing double-cuffed catheters, the internal cuff is placed in the rectus muscle and the external cuff is placed in the subcutaneous tissue proximal to the exit site. Implanted catheters (Fig. 19-1) consist of an intraperitoneal segment containing side holes and an open tip for fluid flow; a subcutaneous segment that passes through the peritoneal membrane, muscles, and subcutaneous tissues; and an external segment that extends from the external cuff to the exit site. There are several versions of chronic catheters, including the Tenckhoff, column disk, Toronto Western, Swan neck, Cruz, and Moncrieff-Popovich catheters (Fig. 19-2). All versions include features intended to improve dialysate flow and decrease catheter complications.

Figure 19-1 Placement of double-cuffed Tenckhoff catheter.
(From Ash SR, Carr DJ, Diaz-Buxo JA: Peritoneal access devices. In Nissenson AR, Fine RN, Gentile DE, editors: Clinical dialysis, ed 2, Norwalk, Conn, 1990, Appleton & Lange.)
What is meant by catheter break-in?
Catheter break-in is the period after the chronic catheter is placed, during which there is healing and tissue ingrowth into the cuff(s). The goals are to promote healing and prevent complications such as dialysate leaks, infections, and catheter obstruction. Healing may take up to six weeks and includes scab formation, granulation of tissue at the exit site, and epithelialization of the sinus tract. Full-volume dialysis, especially CAPD, should be avoided for at least 10 to 14 days to allow healing to occur. This healing period may necessitate the placement of a temporary hemodialysis access if the patient is severely uremic or is fluid overloaded and in need of immediate dialysis. Postoperatively the patient should remain supine when possible and avoid activities that increase intraabdominal pressure, such as straining to defecate, excessive coughing, crying, and lifting. Treatment options during the postoperative period include the following:
• Infusion of heparinized saline solution (1 to 10 units heparin/mL saline), 25 to 100 mL every 4 to 8 hours for 1 to 3 days postoperatively. This protocol will not detect catheter malposition or outflow problems.
• Low volume in and out exchanges with heparinized saline or dialysate done several times a day until the effluent is no longer bloody, then done daily for one to two weeks and weekly thereafter until the patient is on PD. A small volume of heparinized solution should remain in the peritoneum to inhibit the formation of fibrin (whitish protein formed in response to bleeding, inflammation, or infection) and to prevent the development of adhesions. There is no systemic anticoagulation from the administration of low-dose intraperitoneal heparin.
• Low-volume dialysis in the patient who needs immediate dialysis but who is unable to undergo hemodialysis. Frequent, low-volume exchanges (500 to 1000 mL) are performed using a cycler with the patient in the supine position. The volume is gradually increased to eliminate the signs and symptoms of uremia.
How is the adequacy of peritoneal dialysis determined?
The efficiency of PD depends on the ability of the peritoneal membrane to ultrafiltrate fluid and solutes. Peritoneal clearance of solutes is determined by diffusion, which is driven by the concentration gradient between dialysate and plasma and by the “solute drag” created by hypertonic dialysate. Solutes and fluid may move from the intravascular compartment to the peritoneal cavity or from the peritoneal cavity to the intravascular compartment. Other substances lost in the effluent include protein (8.8 to 12.9 g/day), amino acids, water-soluble vitamins, trace minerals, and certain hormones.
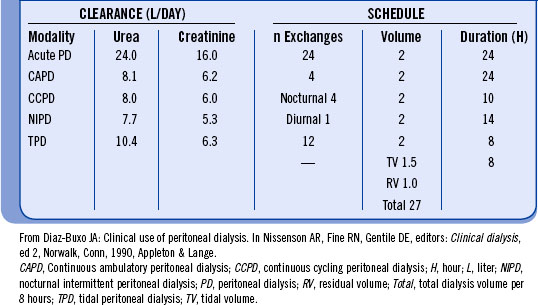
UF and solute clearance in PD are influenced by (1) permeability of the peritoneal membrane, (2) volume of the exchange, (3) dialysate glucose concentration, (4) dwell time, and (5) molecular size of the solute. Clearance of solutes is expressed as the dialysate/plasma (D/P) ratio at a given point in time during the exchange. Equilibration is achieved when the D/P ratio approaches 1. Small solutes, such as urea (molecular weight = 60 Da), are highly diffusible and approach equilibration at four hours. Creatinine (molecular weight = 113 Da) moves more slowly toward equilibration, never reaching it during the typical four-hour CAPD exchange. Table 19-2 demonstrates solute clearances that can be achieved with different types of peritoneal dialysis.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree