During the pseudoglandular period, there is a differentiation of the primitive airway epithelium (Post & Copland, 2002). At the time of branching, the bronchi are enveloped in the mesenchyme that develops into connective tissue, smooth muscle, and cartilaginous rings, among other things. By the end of the 16th week, the bronchial tree is developed without further formation of airways.
During the canalicular stage, further branching of the bronchioles leads to respiratory bronchioles. The lobules of the lungs start to form, and there is a decrease in interstitial tissue. The differentiation of cuboidal epithelium into types I and II pneumocytes begins at this time (DiFiore & Wilson, 1994). Cartilage starts centrally and proceeds peripherally, ending around the 25th week. It is at this point that the number of bronchial generations with cartilage is the same as the adult lung.
The saccular phase is the period when there is growth of the pulmonary parenchyma, continued development of the surfactant system, and a reduction in the connective tissue between the airspaces. During the alveolar period, branching becomes more extensive. There is an exponential increase in the surface area as saccules, which will eventually form alveoli, develop (Burri, 1984). It is during this time that surface epithelium and blood vessels come into even closer contact, allowing for the future exchange of gases. This process continues for at least 3 years after birth.
The fetal lung is the main source of amniotic fluid in the uterus. Abnormal lungs secondary to poor intrauterine growth are among the many abnormalities associated with a low amount of amniotic fluid. Type II pneumocytes appear in the alveolar epithelium and begin to function around the 24th week of life. These cells go on to produce a surfactant, which is a mixture of phospholipids and proteins. The surfactant decreases the surface tension of the lung, allowing for maturation and, upon delivery, expansion of the lung with the newborn’s first breaths. When expectant mothers begin premature labor, administration of glucocorticoids to the mother accelerates the maturation of type II pneumocytes and therefore the production of surfactants (Liggins & Howie, 1972). The molecular properties of surfactants and the physiological role they play is discussed in the section “Surface Tension Properties of the Lung.”
Just before full-term birth, there is approximately 50 mL of surfactant-rich fluid in the lung. This fluid is removed by a combination of active channel transport, lymphatic drainage, and the physics of natural childbirth. Most of the protein-rich portion of this fluid is removed by the lymphatic system. At birth, expansion of the chest cavity with high negative intrathoracic pressures pushes the fluid from the alveoli into the interstitium, then onto the lymphatic channels. By the end of the first few hours after birth, the majority of fluid that was in the lungs during the 9 months of gestation is replaced by air.
During gestation, the lungs do not function as the source of oxygen for the developing organs of the body. That task is completed by the placenta. As a result, the lungs receive poorly oxygenated blood in comparison to the more “vital” fetal organs (see Figure 1.2).
Figure 1.2 (a–c) Fetal circulation and changes at birth. The boxes between parts (a) and (b) describe the fate of certain fetal structures once postnatal circulation is established.
Reprinted from Tortora and Derrickson (2009), with permission from John Wiley & Sons, Inc.
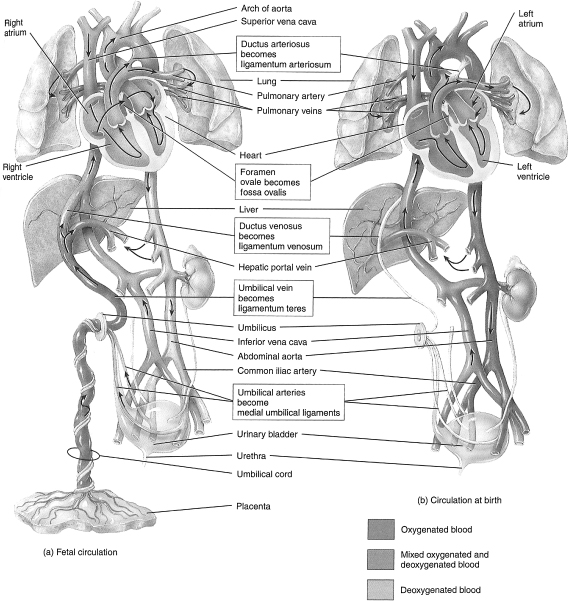
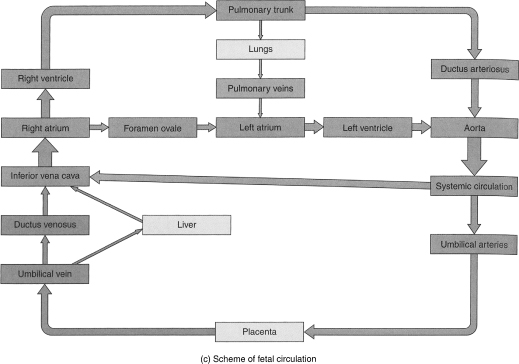
Fetal circulation receives highly oxygenated blood from the placenta through the liver to the right side of the heart. Most of the blood is shunted through the foramen ovale to the left side of the heart. It then either flows through the ascending aorta to the head or through the descending aorta to the systemic circulation. The venous blood that returns from the head flows through the right side of the heart to the pulmonary artery. However, most of this blood is shunted via the ductus arteriosus into the descending aorta to the systemic circulation and the lower part of the body. That blood which does not go through the ductus arteriosus is the blood that oxygenates the lungs. Again, it needs to be pointed out that this blood has already passed through the brain, an organ requiring large amounts of oxygen. As a result, 9 months of this poorly oxygenated circulating blood results from and contributes to the high pulmonary vascular resistance in utero.
Replacement of lung fluid by air upon delivery and breathing by the newborn contributes to the decrease in the pulmonary vascular resistance. The subsequent increase in the partial pressure of oxygen (PO2) and the decrease in the partial pressure of carbon dioxide remove stimuli for vasoconstriction. All of this occurs in association with the reversal of the right-to-left shunt through the ductus arteriosus and foramen ovale present in utero. The ductus closes completely during the first few days after birth.
Pulmonary Vascular Development and the Tracheobronchial Tree
There are two types of airways based on structure and function: (1) cartilaginous airways (bronchi), which make up the conducting system, and (2) membranous, noncartilaginous airways (bronchioles) (see Figure 1.3). The bronchi and nonrespiratory bronchioles serve as conductors of the gas stream, while the respiratory bronchioles and alveolar ducts (terminal respiratory units) serve as sites of gas exchange. The cartilaginous rings of the trachea, except for the cricoid, are not complete and occur over the anterior two-thirds of its surface. The posterior portion is membranous and pliable (Johnson, 2008). The conducting airways’ blood supply comes from branches of the bronchial arteries and the terminal respiratory units from branches of the pulmonary arteries.
Figure 1.3 Branching of airways from the trachea: the bronchial tree.
Reprinted from Tortora and Derrickson (2009), with permission from John Wiley & Sons, Inc.
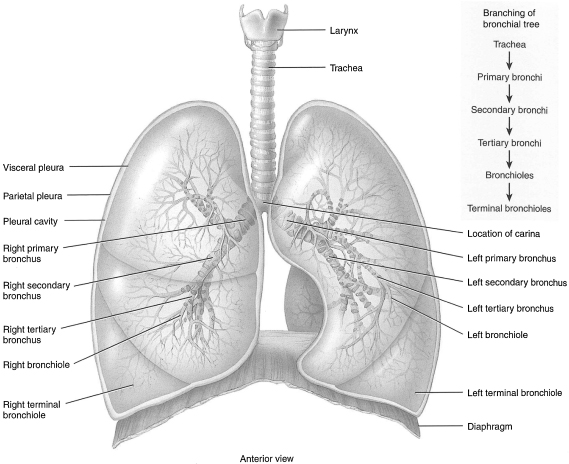
From the trachea to the alveolar sacs, the airway divides 23 times. The first 16 generations form the conducting zone, while the last 7 generations form the transitional and respiratory zone (Sircar, 2008). As stated previously, the conducting airways are in place and are fully formed by birth. However, alveoli are just starting to develop at this point.
While the lung bud is forming, the right and left pulmonary arteries are growing as well; branches of the pulmonary arteries follow branches of the bronchi. By the end of the pseudoglandular period, the conducting airways are complete and have an adult pattern, as are all conventional and supernumerary arteries leading to the terminal respiratory units. The pattern of veins from the sites of gas exchange to the hilum, similar to the conducting airways, is complete halfway through gestation. Conversely, just as alveolar formation continues after birth, the arteries within the terminal unit continue to form for several years after birth.
RESPIRATORY AND NONRESPIRATORY FUNCTIONS OF THE LUNG
Nongas Exchange Functions
The upper airway and tracheobronchial tree do not participate in gas exchange; however, they provide other very important functions. The upper airway warms and humidifies the inspired air and filters out particulate matter. By the time the air reaches the alveoli, it is near body temperature. The hairs in the nostril filter particles larger than 10 µm in diameter. Most of the smaller particles do not travel past the pharynx and are trapped by the tonsils and adenoids. Cilia, which line the respiratory tract down to the terminal bronchioles, pick up any particles between 2–10 µm in size that may have passed through the conducting airways. The cilia are covered in a layer of mucus produced by mucus glands and goblet cells. The cilia move in a synchronized beat carrying these particles up to the larynx where they are swept up to the hypopharynx and swallowed. An abnormality in structure or function of the cilia results in primary ciliary dysfunction, a condition that results in buildup of secretions and a subsequent propensity of bacterial growth in this medium (Johnson, 2008). A similar picture is seen in the lungs of patients with cystic fibrosis, not because of an abnormality of the cilia but because of an abnormality of the mucus layer.
Gases and Gas Exchange
Transfer of Gases Through the Airways
In humans, as the airway branches from the trachea, the branches become smaller and more numerous. Initially from the trachea, the right and left main bronchi divide to lobar (secondary) followed by segmental (tertiary) bronchi and finally to terminal (0.5 mm in diameter) bronchioles. The walls of the primary bronchi are constructed like the trachea, but as the branches of the tree decrease in size, the cartilaginous rings and the mucosa are replaced by smooth muscle. To this point, there are no alveoli. As a result, this portion of the lung is anatomical dead space, as there is no participation in gas exchange. These branches mark the first 16 generations of airways and, based on their main function in the lung, are referred to as the conducting airways. This would be analogous to the branches of a tree (see Figure 1.4).
Figure 1.4 Microscopic anatomy of a lobule of the lungs.
Reprinted from Tortora and Derrickson (2009), with permission from John Wiley & Sons, Inc.
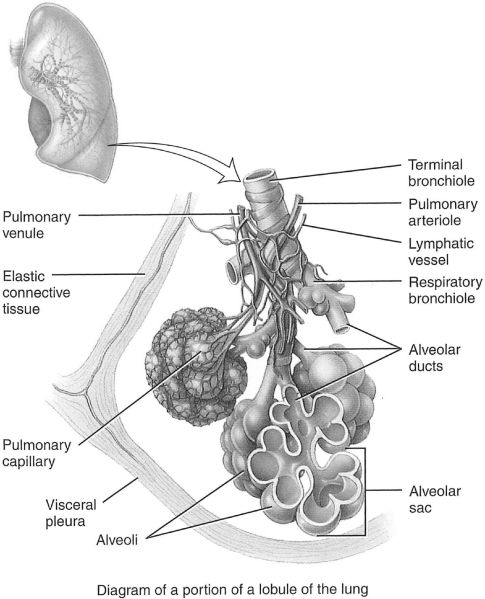
The area where gas exchange occurs is known as the respiratory zone. The occasional alveoli off the walls of the respiratory bronchioles are the first site of gas exchange in the respiratory tree. Alveoli, which is Latin for little cavities, are particular to mammalian lungs. They contain collagen and elastic fibers and are lined with epithelium. The elastic fibers allow the alveoli to stretch with inhalation and retract during exhalation (this is explained in more detail in the section “Control of Ventilation”). Further distal are alveolar ducts and alveolar sacs, which are completely lined with alveoli. Harkening back to the earlier analogy, the respiratory zone would be the leaves on the tree.
Each human lung contains about 300 million alveoli. Each alveolus is wrapped in a fine mesh of capillaries. The alveoli in the respiratory zone of the lungs constitute a total surface area of about 75 m2 (Pavelka & Roth, 2005). The gas exchange tissue is referred to as the pulmonary parenchyma, while the nonparenchyma consists of the conducting airways, lymphatics, noncapillary blood vessels, and the supplying structures.
Transfer of Gas in the Parenchyma
Diffusion
Inspired fresh air moves via bulk movement through the conducting airways. Here, different gases move together along a total pressure gradient. Because of the vast branching of the bronchi and the subsequent increase in the cross-sectional area of the respiratory zone, the velocity of the bulk flow rapidly decreases. As a result, once in the respiratory zone, further movement occurs via diffusion. Diffusion, the movement from an area of high to low pressure, is the principle by which oxygen and carbon dioxide move in different directions between the air and blood. Unicellular organisms do not require respiratory structures for diffusion. However, for more complex organisms, specialized organs have evolved, including gills in fish and lungs in humans (Rhoades & Planzer, 1996). In all these organisms, the underlying basis for the diffusion of gas is the presence of thin walls and a rich supply of blood vessels to allow for exchange and transport of gases.
Different gases move according to each of their partial pressure gradients. From the alveolar sacs, oxygen diffuses into the pulmonary capillary blood. An important concept to discuss here is Fick’s law, which describes diffusion through tissues. Essentially, the rate of transfer of a gas across a surface is directly proportional to the product of the tissue surface area and the difference in gas partial pressure between the two sides of a tissue and is inversely proportional to the thickness of the tissue:
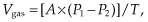
where
| Vgas | = volume of gas diffusing through a tissue per time; | |
| A | = surface area of the tissue; | |
| P1 − P2 | = partial pressure difference of gas across the tissue; and | |
| T | = thickness of the tissue. |
Based on these relationships, it becomes clear how the human lung is ideal for gas transfer: The gas exchange surface is very thin (0.2–0.5 µm) and the surface area vast.
After diffusing through the alveolar–capillary interface, that is, the air–blood barrier, the oxygen molecule enters the red blood cell in the pulmonary capillary, combining with hemoglobin (Hb). The exchange of oxygen from the alveolus into the pulmonary capillary blood occurs rapidly with the pressures equalizing in the two areas in 0.25 second. However, the blood requires 0.75 second to move through the pulmonary capillary bed under resting conditions. Approximately 85–95% of the alveolar surface is intertwined with pulmonary capillaries, with 280 billion capillaries supplying 300 million alveoli (Levitzky, 2007).
Because the PO2 in a red blood cell entering a pulmonary capillary equals the PO2 in the alveolus one-third of the way through the capillary, the transfer of oxygen is defined as perfusion limited. In other words, the amount of oxygen taken up by the blood is dependent on the amount of blood flow through the capillaries. The fact that under normal circumstances oxygen transfer is perfusion limited does not do justice to the enormous diffusion reserve of the human lung. The PO2 in the red blood cell at the initial portion of the pulmonary capillary is 40 mmHg. Across the thin alveolar wall, the PO2 is 100 mmHg. Now, applying Fick’s law, consider the enormous surface area of the respiratory zone, the microscopic thickness of the blood–gas barrier, and the large pressure gradient between the barrier. It becomes obvious under normal conditions why the PO2 in the red cell approximates the PO2 of the alveolar gas in 0.25 second.
As with much of physiology, abnormal conditions, or pathophysiology sheds more light on normal conditions. Perfusion limitation is most often seen in disease states where there is decreased surface area and/or increased thickness of the alveolar–capillary interface. The rate of diffusion will be slowed, but the PO2 of the end capillary red cell is still able to equal the PO2 of alveolar gas. However, with vigorous and prolonged exercise, there is up to a threefold increase in cardiac output and pulmonary blood flow (West, 2003). With the increase in transit time through the pulmonary capillary bed, blood runs through the pulmonary capillaries in less than 0.75 second. In conditions where there is an increased thickness or a decreased surface area, the PO2 in the capillaries is unable to equalize the partial pressure in the alveolus, leading to diffusion limitation. Disease states in which there is an increased thickness of the alveolar–capillary interface include neonatal chronic lung disease and interstitial lung disease. A decrease in the surface area is seen in emphysema (Bates, 1962). In someone without lung disease, the increase in transit time through the pulmonary circulation will not affect end-capillary PO2. Clinically, this is part of the explanation for decreased exercise tolerance in these disease states.
Now take for example persons without lung disease, but place them in an abnormal environment. Again, according to Fick’s law, one of the factors in the diffusion of a gas is the difference in partial pressures. The atmospheric PO2, that is, the pressure that determines the pressure gradient, is assumed to be constant, which at sea level it is. However, the pressure gradient changes in a location of higher altitude. Elevations of 6,500 ft (2,000 m) are classified as high altitude because of this difference in the PO2, but depending on the person, this effect of altitude may be experienced at lower elevations.
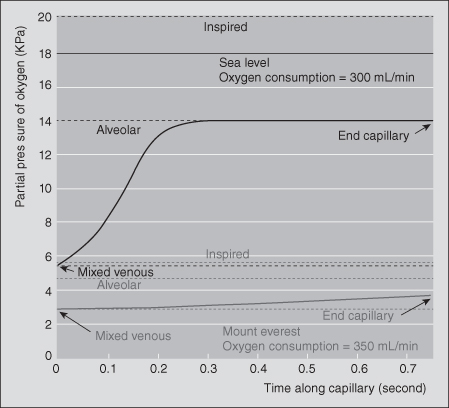
At sea level, oxygen has a partial pressure of 159 mmHg. In Mexico City, it is approximately 125 mmHg, while at the top of Everest, it drops to 48 mmHg (Wilmore & Costill, 2005). Therefore, at the top of Everest, the PO2 is nearly equal to the PO2 at the initial portion of the pulmonary capillary, markedly decreasing the pressure gradient that is the driving force for the transfer of oxygen into Hb (Peacock, 1998) (see Figure 1.5).
Figure 1.5 Calculated time course for change in partial pressure of oxygen in the pulmonary capillary. At sea level, oxygen pressure reaches almost alveolar levels in a third of available time. At the summit of Mount Everest, the mixed venous oxygen pressure is lower and never reaches alveolar levels.
Reprinted from Peacock (1998), with permission from BMJ Publishing Group, Ltd.
The human body attempts to overcome the difference in the pressure gradient of oxygen by controlling what it can, in this case, increasing the transportation capability of oxygen. Erythropoietin (EPO) is a glycoprotein released by the kidney that increases the production of red blood cells in the bone marrow. Within hours at high altitudes, EPO levels increase, and 4 days later, new red blood cells are produced (Harris, Terrio, Miser, & Yetter, 1998). This leads to 75% acclimatization at 7–10 days and 100% acclimatization by 15–20 days. Synthetic EPO has been used by athletes as a performance-enhancing drug to increase Hb and, therefore, oxygen carrying capacity. This has most notably been seen in the Tour de France cycling event.
In individuals accustomed to high altitudes, the concentration of 2,3-diphosphoglycerate (2,3-DPG) in the blood is increased. 2,3-DPG allows these individuals to deliver a larger amount of oxygen to tissues under conditions of lower oxygen tension. This is further explored in the section “Oxyhemoglobin.”
In 1968, the Olympics were held in Mexico City. These were the first games staged at a high altitude (7,349 ft [2,300 m]). The number of world records set and the categories in which they were set in fueled extensive research. The higher altitude led to reduced wind resistance and drag upon the competitors’ bodies. This explained why records in almost every short distance track event from the 100 to 1,500 m were set. However, this was not the case with long-distance events. Athletes in events that involved prolonged aerobic activity and, subsequently, dependency upon maximal amounts of oxygen were adversely affected by the decrease in the atmospheric PO2 (Jenkins, 2005). Because of this, the International Association of Athletic Federations specifically denotes track and field records that have been broken at altitudes greater than 1,000 m.
A significant amount of research was conducted in the wake of the Mexico City Olympics. As a result, the initial United States Olympic Training Center was built in 1978 on an Air Force base in Colorado Springs, Colorado, to take advantage of the physiological principles of high-altitude training.
As stated previously, under normal conditions, the diffusion of oxygen in the human lung is perfusion limited. In contrast, the diffusion of carbon monoxide (CO) is diffusion limited. This is because carbon monoxide bonds very strongly to Hb. This diffusion can occur with a large amount of carbon monoxide without a significant increase in the partial pressure of carbon monoxide (PCO) (Levitzky, 2007).
These gas properties were utilized in developing a pulmonary function test that measures diffusion in the lung. Because the diffusion of oxygen is not measurable, CO is used for testing. This test, the diffusing capacity of the lung for carbon monoxide (DLCO), is based on the high affinity of Hb for CO and on the negligible partial pressure for CO in Hb. Again using Fick’s law, the diffusing capacity of the lung is equal to the volume of carbon monoxide transferred divided by the alveolar PCO. The single-breath DLCO test uses this calculation. A single inspiration of dilute CO is made, and after a 10-second breath hold, the rate of disappearance of CO is measured allowing the difference in partial pressures of inspired and expired carbon monoxide to be determined. The enormity of the surface area of the lung and the inability to measure the thickness of the blood–gas barrier precludes using these two factors in the above-mentioned calculation. However, both of these aspects impact the volume of CO transferred and the PCO. Therefore, abnormalities in these areas will lead to abnormal DLCO measurements.
Conditions that decrease alveolar surface area and therefore decrease DLCO include (1) uneven distribution of oxygen in the lung as seen in emphysema and (2) lung injury secondary to bleomycin administration. Conditions that increase the alveolar–capillary barrier and therefore decrease DLCO include (1) interstitial or alveolar edema and (2) interstitial or alveolar fibrosis as seen with vasculitis, such as sarcoidosis and scleroderma. Conditions that increase DLCO include (1) polycythemia, (2) increased pulmonary blood volume as occurs in exercise or congestive heart failure, and (3) alveolar hemorrhage. The increase in Hb available to bind CO is common to all of these conditions, explaining higher DLCO measurements.
TRANSPORT OF GAS IN THE RED BLOOD CELL
Oxyhemoglobin
The movement of oxygen from the outside environment down the respiratory tract into the alveoli has been discussed. The following explains the mechanisms by which oxygen moves from the alveolus into the pulmonary capillaries and, more specifically, the physiology of the binding of oxygen to Hb.
Heme is an iron compound that is bound to globin, a protein made up of four polypeptides. The iron atom at the center of the heme group is bound to four symmetrically arranged pyrroles and one of four polypeptide chains. The combination of alpha and beta polypeptide chains and the difference in amino acid sequences determines the type of Hb, including normal adult hemoglobin (HbA), fetal hemoglobin (HbF), and hemoglobin S (HbS) (sickle), among others. Each of the four polypeptide chains can bind a molecule of oxygen, though with different affinity (Levitzky, 2007).
HbF has two alpha chains and two gamma chains and has a higher affinity for oxygen than does normal HbA, which has two alpha chains and two beta chains. This distinction is important for oxygen transportation from the placenta in utero, where HbF is the predominant form. Beta chains begin to be produced toward the end of the saccular phase, approximately 6 weeks before birth. HbF all but disappears from circulation by 4 months of age in the infant without hemoglobinopathies.
Oxygen combines with Hb to form oxyhemoglobin. One gram of Hb can combine with 1.39 mL of oxygen (Dominguez de Villota, Ruiz Carmona, Rubio, & de Andrés, 1981). However, some Hb exists as methemoglobin, or is combined with carbon monoxide. Therefore, the oxygen capacity, the maximum amount of oxygen that can combine with Hb, under normal conditions, is 1.34 mL O2/g Hb. Using the example of a person with an Hb concentration of 15 g/dL, the oxygen carrying capacity would be
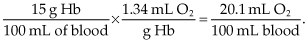
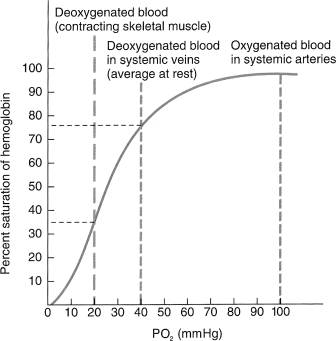
The oxygen saturation of Hb is the amount of oxygen attached to Hb divided by the oxygen capacity. The normal oxygen saturation for arterial blood is over 97%, while in venous blood, it is about 75%.
Oxygen in the blood is transported in two forms: bound to Hb and dissolved in the plasma. While the majority of the oxygen is bound to Hb, a small proportion is dissolved, with the amount dependent on the PO2. For each 1 mmHg PO2, there is 0.003 mL O2 dissolved in 100 mL of plasma. Therefore, the total oxygen concentration of blood (mL O2/100 mL of blood) is equal to the summation of these two forms:
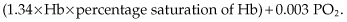
These relationships explain how one can have normal saturation of Hb and PO2 but still have a low oxygen concentration of blood if there is severe anemia.
The relationship of the PO2 of plasma and the Hb saturation is represented graphically by the oxygen dissociation curve (see Figure 1.6).
Figure 1.6 Oxygen–hemoglobin dissociation curve showing the relationship between hemoglobin saturation and PO2 at normal body temperature.
Reprinted from Tortora and Derrickson (2009), with permission from John Wiley & Sons, Inc.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


