11. Patients requiring ophthalmic surgery
Biddy Knight and Sue Hart
CHAPTER CONTENTS
Introduction163
Structure of the eye163
Assessment of the eye168
Preoperative assessment170
Pre- and postoperative care of patients undergoing intraocular surgery170
Pre- and postoperative care of patients undergoing extraocular surgery188
Discharge planning194
Conclusion195
At the end of the chapter the reader will be able to:
• understand the basic structure and function of the eye
• understand the causal relationship between altered physiology and patients’ visual problems
• demonstrate an awareness of the special needs of patients undergoing ophthalmic surgery
• recognize the importance of continuity of care between hospital and the community
• acknowledge the need for effective patient education.
Introduction
Nursing patients with a real or potential visual handicap requires perception, patience and good communication skills as well as the implementation of good nursing care. The majority of elective ophthalmic surgery is carried out on a day care basis and under local anaesthesia.
Individual ophthalmic units have their own protocols regarding patient selection for day care or overnight stay, and there should be a specialist facility for those who need longer periods of care. The Royal College of Ophthalmologists (2005) recommend that all units have inpatient facilities that are separate from other surgical activity, so reducing the risk of infection.
The medical and nursing care that patients receive will also vary from unit to unit, and the following discussion is intended as a guide to key principles of care.
Many patients requiring ophthalmic surgery are in the older age group and may have other medical conditions; these facts must be considered when planning their admission, care and discharge.
Surgery can take many forms, because within the eye there are many structures that affect vision and require surgical intervention to correct or halt a decrease in visual acuity. Knowledge of the structure and function of the eye and its component parts aids understanding of the abnormalities that can occur and the operations that are performed.
Structure of the eye
Both eyes consist of a globe, cushioned by orbital fat within a cone-shaped bony orbit, and protected anteriorly by the lids, lashes and tear flow.
Sclera
The sclera is an opaque, dense layer of tough fibrous tissue with a high collagen content which prevents light entering the eye inadvertently. The opaque nature of the sclera is due to the haphazard positioning of the collagen fibres. It is 0.6–1 mm thick except at the insertion of the recti muscles, where it is only 0.3 mm in depth. The blood supply comes from the posterior ciliary arteries in the elastic episclera that covers the sclera. The nerve supply is derived from the ciliary branch of the oculomotor nerve.
The weakest part of the sclera is the posterior area where it is pierced by the optic nerve fibres, resulting in a sieve-like structure known as the lamina cibrosa, and where the sclera becomes continuous with the dural layer of the meninges. Anteriorly, the sclera merges with the cornea at the limbus. The sclera is a protective layer; it is more elastic in children, becoming tougher with increasing age.
Cornea
The cornea constitutes the anterior one-sixth of the eye; it is 0.5 mm thick centrally and thicker at the periphery; it averages 12 mm × 11 mm in adults. It is a transparent layer with a convex anterior curve, allowing the passage of light rays to focus on the retina.
It is divided into five layers:
• Epithelium is composed of five to six layers of squamous stratified epithelium which is continuous with the epithelium of the conjunctiva, and is the only regenerative layer. Damage results in bacteria being able to penetrate corneal tissue.
• Bowman’s membrane is the anterior elastic membrane, consisting of a thin layer of collagen which is tough, forming a protective layer that does not regenerate if damaged, resulting in scarring.
• Stroma accounts for 90% of corneal tissue and consists of modified collagen fibres and keratinocytes.
• Descemet’s membrane is the posterior elastic membrane, acting as a barrier against invasion by micro-organisms, chemicals and changes in intraocular pressure.
• Endothelium is a single layer of cells that lines the posterior surface of the cornea and ‘pumps’ fluid from the cornea into the anterior chamber. Interference with this function results in corneal oedema and loss of transparency.
The cornea is essentially avascular; nutrition and oxygen is obtained from the vascular arcades of the anterior ciliary arteries at the limbus, the aqueous via diffusion at the endothelium and from atmospheric oxygen. Corneal nerve supply is derived from the ophthalmic division of the trigeminal nerve.
The cornea has two functions:
• protection
• refraction: it is the most powerful refractive part of the eye, and essential to this function is corneal clarity, which is maintained by:
– avascularity
– uniformity of structure
– efficient epithelial function.
Iris
The iris is a pigmented disc with a central opening, the pupil. It is situated in front of the lens and behind the cornea, so separating the anterior and posterior chambers, and is a forward extension of the ciliary body.
There are three layers:
• endothelium
• stroma, consisting of pigmented cells, blood vessels, nerves and muscles
• pigmented epithelium, continuous with the pigmented epithelium of the retina.
The colour of the iris results from the presence of melanin and is genetically predetermined. Initially, babies only have pigment in the epithelial layer, but, over the first few weeks of life, pigment is laid down in the stroma and the eyes acquire their adult colour.
There are two muscle groups in the iris: the radial dilators and the central sphincter constrictor, the latter being the more powerful. The nerve supply is derived from the short ciliary branch of the oculomotor nerve for the sphincter pupillae and from the long ciliary branch of the trigeminal nerve for the radial pupillae. The arterial capillaries of the long posterior ciliary arteries and the anterior ciliary arteries join to form a circular vascular network.
Ciliary body
The ciliary body is triangular, lying between the choroid and the iris. It is continuous with the iris and has numerous folds on its inner surface, the ciliary processes, where secretion of aqueous takes place.
Most of the ciliary body consists of circular and longitudinal muscle fibres whose function is to alter the shape of the lens, i.e. accommodation, by exerting an equal force on the suspensory ligaments that run from the margin of the ciliary body to the periphery of the lens.
The ciliary body can be divided into three areas:
• The pars plicata contains the 70–80 radiating strips that constitute the ciliary processes and secrete aqueous into the posterior chamber.
• The pars plana is continuous with the pars plicata.
• The ciliary muscles are situated on the anterior surface of the ciliary body and are composed of circular and longitudinal fibres. They contract and relax to bring about accommodation, resulting in light rays being focused on the retina. Contraction of the ciliary muscles results in the relaxation of the suspensory ligaments, and the lens becomes more bulbous, so increasing refraction, as when viewing near objects.
The nerve supply is derived from the short ciliary branch of the oculomotor nerve. The blood supply is the long posterior ciliary artery and vein, the anterior ciliary artery and vein, and the vortex vein. The ciliary body produces and secretes aqueous, as well as altering the shape of the lens.
The choroid
The choroid is a pigmented, highly vascular layer lying between the sclera and the retina. It extends from its junction with the ciliary body, the ora serrata, posteriorly to the optic disc.
It is composed of four layers:
• suprachoroid – contains elastic tissue, pigment cells and collagen
• vascular layer – large and small blood vessels supported within a pigmented stromal tissue
• choriocapillaries – capillaries
• Bruch’s membrane – a protective, supporting sheath.
The nerve supply is derived from the posterior ciliary branch of the oculomotor nerve. Blood supply is from the short posterior ciliary artery and is drained away by the choroidal and vortex veins.
The choroid provides nutrients for retinal cells adjacent to the choroid, especially the rods and cones. The pigment in the choroid prevents light rays scattering and causing internal reflection of light, so aiding focusing of light rays on the retina.
The retina
The retina is a complex structure consisting of 10 layers of cells, divided into two separate parts. One part is made up of nine layers, the transparent neural division; this lies on the single pigmented epithelial layer which is adjacent to the choroid (Stollery et al, 2005). These two parts are firmly attached to each other only at the optic disc and the ora serrata.
Three specific areas of the retina must be considered:
• The macula lies in the central area of the retina, 3 mm to the temporal side of the optic disc, and is 1.5 mm in diameter. It consists mainly of cones, and in its centre is the fovea, an area consisting entirely of cones. Macular function is to give very precise, coloured, central vision, and it lies on the visual axis.
• The rest of the retina consists of a mix of cones and rods, the latter being responsible for the perception of light and dark.
• The optic disc is found at the point where the retinal veins and nerve fibres leave the eye and the retinal artery enters. There are no light receptors in this area, so it is insensitive to light and is consequently known as the ‘blind spot’.
• Once a nerve impulse is initiated, it is transmitted along the visual pathway, via the optic nerve to the occipital cortex. Impulses from the nasal fibres of each eye cross at the optic chiasma to the branch of the optic nerve on the other side of the brain.
• The ora serrata is the anterior edge of the retina, where the retinal pigmented layer merges with the ciliary epithelium and the neural layers end.
The blood supply of the retina is derived from two main sources: the anterior one-third is from the choriocapillaries of the choroid, and the posterior two-thirds is from the central retinal artery. The retina is one of the few areas in the body where the blood vessels can be viewed directly.
The retina reacts to the presence of light and initiates impulses that are then transmitted to the visual cortex of the brain for interpretation.
The transparent media of the eye
This consists of the cornea plus the aqueous and vitreous humours and the lens.
Aqueous fluid
Aqueous fluid consists mainly of water, with some proteins and chlorides, and is produced by the ciliary processes of the ciliary body. It is secreted into the posterior chamber and flows around the lens, through the pupil and circulates around the anterior chamber before draining via the trabecular meshwork into the canal of Schlemm and so into the venous return of the eye. Aqueous also drains out through the ciliary body into the episcleral vessels, i.e. the uveal scleral route. The openings to the trabecular meshwork are located in the drainage angle of the anterior chamber, formed by the junction of the cornea and the iris.
The aqueous is responsible for maintaining intraocular pressure at approximately 15–20 mmHg (Stollery et al, 2005). It nourishes the lens and posterior surface of the cornea and provides a clear medium for refraction.
Lens
The lens is a biconvex structure approximately 9 mm × 4 mm, lying between the posterior surface of the iris and the anterior surface of the vitreous. It is avascular, is not innervated, and is held in position by the suspensory ligaments or zonules.
It is composed of three parts: the elastic capsule, epithelial cells on the anterior surface, and the lens substance. The lens substance consists of a nucleus, layers of protein called crystallins arranged like an onion and ‘Y’ sutures that mark the junction of the protein fibres. Nutrition is provided by the aqueous, and the crystallins act as enzymes to convert sugar into energy. Depending on the position of the object being viewed, the lens ‘accommodates’, so allowing the light rays to focus on the retina.
Vitreous
Vitreous lies between the posterior capsule of the lens and the retina, in the posterior cavity, within the hyaloid membrane. The vitreous body consists of a semigelatinous substance that is produced during embryonic life and, if lost, cannot be replaced naturally. It is avascular, is not innervated, and receives nutrition from the blood vessels of the choroid, retina and ciliary body. It is attached to the ciliary body at the ora serrata and to the retina at the optic disc. The vitreous holds the retina in place, helps to maintain intraocular pressure and preserves the shape of the eye. It also assists in refraction.
Eyelids
The eyelids act as protection for the anterior portion of the eyes, and the epithelium of the lids is continuous with the conjunctiva lining the inner aspect of the lids. Their shape and strength is maintained by cartilaginous tissue forming the upper and lower tarsal plates; within these are found the meibomian glands, which secrete sebum, a substance necessary to control tear flow, lubricate the lid margins and prevent excessive evaporation of tears from the surface of the eye.
Eyelashes are situated along the lid margins and act as protective filters; they are kept supple by sebum secreted directly into the lash follicles by the glands of Zeis.
There are two main muscle groups in the eyelids: a sphincter called the orbicularis oculae, responsible for closing the eye, and the levator palpebrae, whose function is to raise the upper lid. Movement of the lids may be both voluntary and involuntary.
The nerve supply to the orbicularis muscle is from the facial nerve, and the oculomotor nerve supplies the levator palpebrae. The blood vessels to and from the lids are the lacrimal artery and vein, the superior and inferior medial palpebral artery and vein, and the supraorbital artery and vein.
The functions of the eyelids are to:
• protect the eyes from excessive light
• protect the eyes from foreign objects
• lubricate the anterior surface of the eye
• prevent the anterior surface of the eye from drying out, even during sleep.
The conjunctiva
The nerve supply is derived from the nasociliary branch of the trigeminal nerve. There is a rich blood supply from the anterior ciliary artery and vein, the superior and inferior medial palpebral artery and vein, and the conjunctival artery and vein.
The conjunctiva:
• produces the mucin layer of the tear film, so reducing the rate of tear evaporation
• facilitates movement by moistening the surface of the eye and lids
• protects the eye against damage and infection.
Lacrimal apparatus
The lacrimal apparatus consists of the lacrimal gland, ducts, superior and inferior puncta and canaliculi, common canaliculus, lacrimal sac and nasolacrimal duct.
Tears are produced in the lacrimal gland, which is situated in the upper outer quadrant of the orbit, and then drain via the tear ducts onto the anterior surface of the eye. Blinking causes the tears to be distributed across the cornea, towards the puncta at the inner aspect of the eye. They then drain into the canaliculi via the puncta and collect in the lacrimal sac before draining into the nose via the nasolacrimal duct. Tears consist of water, protein, glucose, sodium, potassium, chloride, urea and lysozymes. Mucin from the goblet cells of the conjunctiva, and an oily layer from the meibomian glands, facilitate movements, slow down evaporation and prevent overflow onto the cheeks.
The nerve supply to all parts of the lacrimal apparatus is from branches of the trigeminal nerve. The blood supply to the lacrimal gland is from the lacrimal artery and vein, whereas the rest of the system is supplied by the nasal artery and vein, and the superior and inferior medial palpebral artery and vein.
The lacrimal system produces tears which:
• aid refraction by providing an optically smooth corneal surface
• lubricate the anterior surface of the eye, so easing movement
• clean dust particles from the eye
• protect against infection by the action of lysozymes.
The orbit
The eye is protected by being situated in a pyramid-shaped bony cavity – the orbit. It consists of seven fused bones:
• ethmoid
• sphenoid
• frontal
• lacrimal
• zygomatic
• palatine
• maxilla.
Anteriorly, the orbit is open and its apex is positioned posteriorly. Each orbit is described as having a medial wall on the nasal side, a lateral wall, a roof and a floor, the floor and the lower part of the medial wall being the thinnest.
The orbit contains the eyeball, six extraocular muscles, ophthalmic artery and vein, the optic, oculomotor, trochlea, trigeminal and abducens cranial nerves, lacrimal gland, lacrimal sac, orbital fascia, fat and ligaments. There are three openings within the orbital wall, the largest of which is called the optic foramen, and it is here that the optic nerve and ophthalmic artery enter. The bony nature of the orbit acts as a very effective protection from most trauma, except anteriorly.
Extraocular muscles
There are six muscles concerned with the movement of each eye, and they work together to give the precise coordination of movement that is essential for good vision. They are mainly voluntary, and each one is involved in all ocular movement by a balance of contraction and relaxation.
All the muscles receive their blood supply from the muscular arteries, but innervation differs from muscle to muscle:
• abducens nerve – lacteral rectus muscle
• trochlea nerve – superior oblique muscle.
Assessment of the eye
Whenever anybody presents with an abnormality of vision, no matter how trivial it may seem, it can cause pain and a fundamental fear of losing sight. This means that the nurse has to use all their psychological skills to reassure the individual and gain their cooperation in order to make an accurate assessment of their vision and the condition of their eye. All information gained should be clearly documented, as a written recording of all findings is a legal requirement (Elkington and Khaw, 1999).
The first element of assessment should be the measurement of vision, unless this is not feasible because of the extent of injury, acute pain, or inability to participate, e.g. due to altered level of consciousness. This is done for medical, legal and diagnostic reasons, as visual acuity is a measure of macular function.
The most common way of testing visual acuity is with a Snellen’s chart. This requires the patient to read letters of varying sizes on a chart 6 m away (this excludes all but a very small amount of accommodation). The letter size indicates at what distance a normal-sighted person should be able to see it:
• 6/60: normal-sighted person would see this 60 m away
• 6/6: normal-sighted person would see this 6 m away.
In younger people especially, vision may be better than this: e.g. 6/5, 6/4. However, if 6/60, being the largest letter on the chart, cannot be seen, then the person’s ability to count the fingers on a hand held up a metre away (CF) or the appreciation of movement (HM) is recorded. If this is not achieved, then perception of a light source is determined and recorded as perception of light (PL) or no perception of light (NPL).
Each eye should be tested individually, the worst eye first, as there may be a degree of unconscious recall. A record should be made of whether glasses or contact lenses are being worn, as an apparent discrepancy in visual acuity may occur when it is recorded next time.
Improvement in visual acuity may be achieved by using a pinhole: the patient looks at the Snellen’s chart through a small pinhole, which means that light only passes along the principal visual axis of the eye and so vision is less affected by any abnormality of refraction.
Assumptions must not be made about the patients’ ability to read, especially if they appear to be able to see effectively, i.e. they have negotiated their way around obstacles with ease. They may be reluctant to admit they cannot read, but this can be overcome diplomatically by using a Snellen’s chart based on the letter ‘E’ or with pictures rather than letters. Alternatively, use of a LogMAR chart (logarithm of the minimum angle of resolution) gives a more effective and precise record of visual acuity.
Near vision is tested using ordinary printer’s type. Colour vision can be assessed using the Ishihara colour plates.
Visual acuity should always be measured before instilling mydriatic drops, as many of them also have a cycloplegic effect and so paralyse the ciliary muscle and alter accommodation. This also applies when assessing a patient’s visual field. If visual acuity is measured following instillation of mydriatics, this must be recorded in the notes.
A basic assessment of visual field can be done by asking the patient to say when they can see an object moving in from the side while focusing on a central object. More precise results can be obtained by using a perimetry machine, which plots peripheral vision by moving a target across a semicircle marked out in degrees. Computerized field analysers are also capable of interpreting results related to the intensity of light needed to stimulate retinal activity.
Mydriatics should not be used when raised intraocular pressure is suspected, as dilating the pupil decreases the amount of aqueous being drained and will increase intraocular pressure even more.
Intraocular pressure is measured most accurately by the use of an applanation tonometer attached to a slit lamp; as this requires the applanator head to be pushed against the cornea, local anaesthetic drops should always be instilled prior to use. Patients who cannot be positioned at a slit lamp can have their intraocular pressures measured using a handheld tonometer, such as a TonoPen. If this apparatus is not available, the presence of raised intraocular pressure may be identified by means of careful digital palpation, but this must be done with great care and never when a perforating injury is suspected.
Care must be taken to continue the systematic examination by beginning with the outer aspect of the eye and then going on to the internal structures, to ensure an accurate assessment of the condition of the eye.
• Conjunctiva – observe for lacerations, degree and position of vascular injection, oedema (chemosis), foreign bodies, naevus, pinguecula and pterygium.
• Cornea – note any lacerations, foreign bodies, surface anomalies and the degree of corneal clarity. Identification of superficial damage may be assisted by the instillation of fluorescein sodium 2% drops, as the damaged area will then appear bright green when illuminated. Rose bengal 1% drops may also be used, as they stain all dead corneal tissue pink. If the patient complains of seeing haloes around lights, this may indicate the presence of corneal oedema.
• Anterior chamber – shining the beam of light in at an angle makes it is possible to estimate the depth of the anterior chamber. It is also important to note whether the chamber is clear or contains red or white cells and, if these are present, whether they are settled with a specific level or diffuse throughout the chamber.
• Iris – examine for any obvious bleeding points or any abnormal pigmentation, and ensure that it is moving freely and equally in all directions, is intact and in position.
• Pupil – examine both pupils to verify the existence of a consensual response and equality of size. Their shape, size and briskness of movement are also recorded. The pupil should be black in colour unless there is a reflection from the retina, in which case the pupil appears red. Sometimes the pupil may appear white, possibly indicating the presence of a cataract.
• Retina – the optic disc may be examined using a direct ophthalmoscope through a normal-sized pupil, but in order for the whole retina, especially the periphery, to be examined, the pupil needs to be dilated using mydriatic drops. The most efficient way of then examining the retina is with an indirect ophthalmoscope (Fig. 11.1).
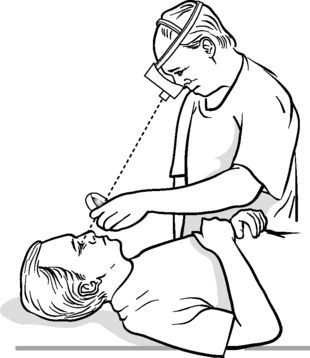 |
| Figure 11.1 • Indirect ophthalmoscopy. |
Apart from the overall examination of the eye, more specific investigations may be carried out.
• Keratometry: measurement of the curvature of the cornea.
• Fluorescein angiography: injection of fluorescein dye, which allows the retinal vessels to be visualized.
• Gonioscopy: examination of the drainage angle.
• Biometry: measurement of the axial length of the eye.
• Ultrasound: measurement of blood flow and exclusion of retinal detachment/tear or tumour in the presence of opacity in the transparent media obscuring direct observation.
• Corneal topography: maps the corneal surface.
• Pachymetry: measures the corneal thickness.
• Amsler grid test: monitors macular degeneration.
• Exophthalmometry: measures degree of proptosis.
• Refraction: determines strength of refractive elements of the eye.
Preoperative assessment
Most units require patients having day surgery to fulfil certain criteria (see Chs 1 and 3). If the criteria are met, the patient’s medical condition is monitored for suitability for local anaesthesia.
• The patient must be able to cooperate: for example, be capable of lying down and keeping still for a period of 20–40 minutes.
• Any medical condition, e.g. diabetes mellitus or hypertension, must be well controlled.
Specific ophthalmic investigations will depend on the type of surgery being undertaken. For cataract surgery with lens implant, keratometry will be undertaken to measure the curvature of the cornea, and biometry to measure the length of the eye’s axis. These two measurements allow the required strength of the implant to be determined. If the patient requires surgery for glaucoma, a series of recordings of their intraocular pressure may be taken. This is known as phasing and usually takes place over a 12-hour period.
Careful observation of the eyes should be made, especially with patients having intraocular surgery, as any local infection must be treated prior to admission. These assessments normally take place 2–4 weeks prior to admission, and any community support required postoperatively needs to be identified and arranged at this stage.
Pre- and postoperative care of patients undergoing intraocular surgery
Care needs to be planned systematically, usually through the use of an integrated care pathway (ICP). Planned ophthalmic surgery lends itself to ICPs, as the patient’s journey is usually clearly defined and so fits Middleton et al’s (2000) definition of an integrated care pathway:
A multidisciplinary outline of anticipated care, placed in an appropriate timeframe, to help a patient with a specific condition or set of symptoms move progressively through a clinical experience to positive outcomes.
Well-designed ICPs also make clear links between locally delivered care and the best-available evidence base (Clark, 2003), and may also reflect key principles of nursing models such as that of Orem (2001), which emphasizes self-care, or Roper, Logan and Tierney (1996), which sees surgical intervention as an episode in life and looks for the actual and potential problems related to the activities of living.
The areas identified in the discussion on pre- and postoperative care are only meant as a guide (Table 11.1 and Table 11.2), and focus on patients having their surgery under general anaesthesia, but they can be adapted to take into account the type of anaesthesia, medical condition, degree of visual handicap and social background. The patient and carers should be involved if they wish, and the design and use of ICPs/care plans should take into account that the patient may have difficulty reading, so time will be needed to discuss it with them.
| Patient’s problems | Intervention | Outcome |
|---|---|---|
| Communication | ||
| New surroundings and unfamiliar routine | Orient patient and family to unit and explain expected course of events up to and including discharge | Well-oriented, relaxed patient; informed patient and family |
| Decreased vision and possible loss of independence | Explain that surgery may improve/maintain vision | Patient optimistic about outcome of surgery |
| Fear of anaesthesia and surgery | Explain exactly what is going to happen prior to, during and after surgery | Patient understands events |
| Maintaining a safe environment | ||
| Potential risk of wrong operation | Check consent form has been signed and understood by patient | Correct operation performed |
| Potential risk of peri- and postoperative complications | Instil prescribed topical medication such as miotics, mydriatics or antibiotics Give prescribed systemic premedication Follow unit’s preoperative protocol Check for any allergies, e.g. latex, iodine | Surgery is performed without complications |
| Potential risk of deterioration in a pre-existing medical condition | After consultation with medical/anaesthetic staff, give necessary medication, e.g. insulin, hypotensive agents Initiate supportive therapy, e.g. physiotherapy Facilitate patient’s involvement in own care | No deterioration in medical condition |
| Risk of corneal abrasion due to loss of sensation, secondary to application of local anaesthetic drops | Examine for signs of corneal trauma | Cornea is not abraded |
| Patient’s problem | Intervention | Outcome |
|---|---|---|
| Breathing | ||
| Potential difficulty with breathing | Check that patient is able to maintain own airway Position patient comfortably and encourage effective breathing | Adequate respirations and no acquired chest infection |
| Maintaining a safe environment | ||
| Difficulty with maintaining own safety | Assess orientation of patient; reorientate as required Educate staff and visitors about the importance of keeping things in the same place and avoiding leaving objects around that may cause injury or confusion Inform all appropriate departments and staff of patient’s visual handicap Adjust lighting to suit individual needs; dark glasses may be worn as required | Orientated patient, able to look after self |
| Potential problem of ocular pain/discomfort | Observe for signs of pain/discomfort Offer prescribed analgesics, and monitor effect Report unrelieved or severe pain to the medical staff | Patient states that they are comfortable |
| Potential risk of delayed recovery due to ocular complications | Systematic examination of eye(s) Cleanse eye as required Advise patient not to rub or wipe eye with used handkerchief/tissue If necessary, apply ‘shield’ to eye to avoid inadvertent rubbing Instil, or educate patient/family to instil/administer, prescribed medication | Uneventful recovery |
| Risk of corneal abrasion due to loss of sensation secondary to application of local anaesthetic drops | Examine for signs of corneal trauma | Cornea is not abraded |
| Communication | ||
| Difficulty due to poor vision reducing impact of non-verbal communication | Emphasize the importance of appropriate touch Use tone of voice to reinforce message Approach patient from the side with most vision, and speak as approaching or leaving | Establishes an effective rapport between nurse and patient Patient is not startled |
| Anxiety regarding success | Adequate information is given and questions answered | Patient is aware of outcome |
| Mobilization | ||
| Difficulty with mobilization due to: anaesthesia; visual acuity | Mobilization is determined by operation and surgeon’s preference, but should be as soon as possible Walking aids to be accessible and call-bell within patient’s reach | Preoperative level of mobility restored Patient mobilizes safely |
| Eating and drinking | ||
| Difficulty with eating and drinking due to: poor vision; effects of anaesthesia | Assist with selection of food and dietary intake Observe for signs of nausea Give antiemetic as required | Balanced diet eaten to facilitate healing Patient does not vomit, and subsequent raised introcular pressure does not weaken the incision |
| Working and playing | ||
| Potential problem of being unable to return to normal lifestyle | With the involvement and cooperation of patient and family/carer, an effective discharge plan (see p. 194) is devised and implemented | Patient is confident to return home |
Cleaning the eye
If both eyes require cleaning, they are cleaned one at a time, and if there is any sign of infection, separate packs should be used for each eye, with the potentially infected eye cleaned second.
Patients known to have an infected eye should not have their eyes cleaned until after all other dressings have been completed, and, where possible, cared for by a nurse who is not responsible for any pre- or postoperative ophthalmic patients, to reduce the risk of cross-infection.
Cleaning is carried out using lint/gauze soaked in normal saline 0.9% or cooled boiled water. Sterile packs containing lint/gauze squares, cotton buds, gallipot and a paper towel are usually available. The towel is used either to dry the nurse’s hands after washing or to protect the patient’s clothing. The eye is cleaned using a lint/gauze square folded into four, with the fluffy side of the lint square innermost, to prevent strands being left in the eye, causing irritation. The swab is held by the cut edges, the folded edges being used to actually clean along the lid margin. This further reduces the likelihood of particles being left along the margins.
Lid margins are cleaned from the inner canthus outwards, avoiding any potential infective agents being swept into the lacrimal drainage system via the puncta. Care should also be taken to avoid contact with the cornea, as this may cause the eyes to close involuntarily, due to pain, and may result in corneal abrasion. Each swab is used once and then discarded; four to six swabs are usually sufficient for this procedure.
If an eyepad is required, the eye must be closed and the pad applied firmly, as contact between the cornea and the pad will result in a corneal abrasion. This is especially important if local anaesthetic drops have been instilled.
Instillation of eye medication
Eye medications are prescribed in two main topical forms: drops or guttae (G.), or ointment or oculentum (Oc.).
Eye medication is instilled into the lower fornix, the ‘gutter’, which is formed by gently pulling down the lower lid. Drops are placed in the middle of the fornix, avoiding the puncta, as this can result in a dry mouth and unpleasant taste caused by the drops draining away via the lacrimal system. The eye dropper should not come into contact with the corneal surface, but should not be held too far away, as this increases the force with which the drop touches the eye, causing a sudden reflex squeezing which may increase intraocular pressure and so threaten the integrity of the incision, if present. This may also occur if the drops cause a stinging sensation, so the patient should be warned.
When instilling beta-blocking agents such as timolol, it is advisable to occlude the puncta with a fingertip for 2–3 minutes to reduce the risk of systemic uptake (International Glaucoma Association, 2008a).
Ointments are squeezed gently into the inner aspect of the fornix, being careful not to touch the eye with the nozzle of the tube. Only a small amount should be applied and the eye then closed gently; any excess should then be wiped away. Patients should be made aware that the ointment may create a film across the surface of the cornea and so blur vision.
Where a combination of drops are used, 5 minutes should be left between each to ensure effective uptake (Pavan-Langston and Dunkel, 1991, cited in Marsden, 2007), and in the case of ointments, they should be placed next to each other in the fornix, not on top of each other, as one may be lost when the eye closes. Drops are instilled before ointment, otherwise absorption is reduced.
Contact between the dropper/nozzle and the cornea can result in a corneal abrasion, so any complaints of pain should be noted and the eye examined.
Corneal graft (keratoplasty)
A corneal graft is replacement of scarred or degenerative corneal tissue by healthy tissue. This is a less complicated procedure than organ transplant, because of corneal avascularity, although this may have been compromised by new vessel growth from the corneal margins.
Removal and storage of donor material is subject to the Human Tissue Act (DoH, 2004).
Although most people are suitable donors, there are some exceptions:
• infections such as methicillin-resistant Staphylococcus aureus, HIV, hepatitis A, B and C, syphilis, and septicaemia
• unexplained neurological disease, because of the risk of infections such as Creutzfeldt–Jakob disease
• leukaemia, lymphoma and myeloma
• eye conditions such as uveitis, retinoblastoma, history of intraocular surgery, and malignancies of the ciliary body and iris
• jaundice
• death due to unknown cause, although donation may take place after a postmortem has been carried out.
Types of corneal graft
Partial thickness or lamellar
Anterior lamellar keratoplasty
This is a partial-thickness graft that involves replacement of the abnormal corneal tissue down to the level of Descemet’s layer (Fig. 11.2). It is used when the pathology is anterior to Descemet’s membrane and has the advantage of preserving the healthy endothelium. The risk of rejection is decreased, as the endothelium is not involved (Kanski, 2003).
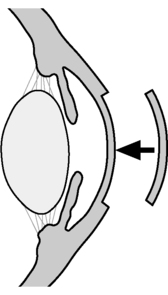 |
| Figure 11.2 • Lamellar keratoplasty. |
Posterior lamellar or endothelial keratoplasty
This is a partial-thickness graft that involves replacement of only the posterior layers of the cornea, namely Descemet’s membrane and endothelium. It is used when the pathology is confined to the corneal endothelium and where the anterior corneal structures are unaffected. The technique is relatively new and has the advantage of quicker visual recovery, increased corneal integrity and reduced risk of rejection. This approach is only available in a few specialist centres and, according to Mearza et al (2007), has a higher graft dislocation and failure rate.
Full thickness or penetrating
This is the replacement of all five corneal layers (Fig. 11.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access

