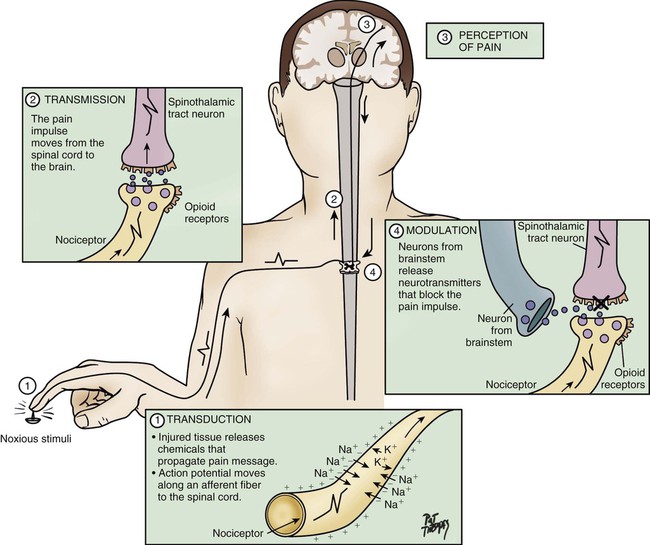
Despite national and international efforts, guidelines, standards of practice, position statements, and many important discoveries in the field of pain management in the past three decades, critically ill patients suffer from moderate to severe pain that can be experienced at rest or during routine care.1,2 For instance, chest tube removal, turning, drain removal, and wound care were identified as the most painful procedures by critically ill adults in previous studies.2–4 Despite this situation, pain remains undertreated in most critically ill patients.5 Poor treatment of acute pain may lead to the development of serious complications6,7 and chronic pain syndromes,8–10 which may seriously impact the patient’s functioning, quality of life, and well-being. Such evidence reinforces the importance of providing attention to pain in this specific context of care. Appropriate pain assessment is the foundation of effective pain treatment. Because pain is recognized as a subjective experience, the patient’s self-report is considered the most valid measure for pain and should be obtained as often as possible.11 Unfortunately in critical care, many factors such as the administration of sedative agents, the use of mechanical ventilation, and altered levels of consciousness may impact communication with patients.12,13 These obstacles make pain assessment more complex. Nevertheless, except for being unable to speak, many mechanically ventilated patients can communicate that they are in pain by using head nodding, hand motions or by seeking attention with other movements.4 In such a situation, use of appropriate communication methods may reduce patients’ distress associated with the presence of the endotracheal tube by enabling them to report the presence of pain or discomfort in a comprehensive way.14 Self-report pain intensity scales have been used with postoperative mechanically ventilated patients who were asked to point on the scale.2,15 However, in a study of mechanically ventilated adults with various diagnoses (trauma, surgical, or medical), only one third of mechanically ventilated patients were able to use a pain intensity scale.15 With a greater degree of critical illness, providing a pain intensity self-report becomes more difficult because it requires concentration and energy. When the patient is unable to communicate in any way, observable behavioral indicators become unique indices for pain assessment and are part of clinical guidelines and recommendations developed in North America.16,17 Pain is frequently encountered in critical care, and there is increased emphasis on the professional responsibility to manage the patient’s pain effectively. The critical care nurse must understand the mechanisms, assessment process, and appropriate therapeutic measures for managing pain. Pain is described as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.18 This definition emphasizes the subjective and multidimensional nature of pain. More specifically, the subjective characteristic implies that pain is whatever the person experiencing it says it is and that it exists whenever he or she says it does.19 This definition also suggests that the patient is able to self-report. However, in the critical care context, many patients are unable to self-report their pain. Some authors20 have proposed an alternative definition for nonverbal patients, in this case infants, but the same principle applies to any non-verbal population, stating that changes in behaviors caused by pain are valuable forms of self-report and should be considered as alternative measures of pain. Based upon this, pain assessment must be designed to conform to the communication capabilities of the patient. The experience of pain includes sensory, affective, cognitive, behavioral, and physiologic components21: The sensory component is the perception of many characteristics of pain, such as intensity, location, and quality. The affective component includes negative emotions such as unpleasantness, anxiety, fear, and anticipation that may be associated with the experience of pain. The cognitive component refers to the interpretation or the meaning of pain by the person who is experiencing it. The behavioral component includes the strategies used by the person to express, avoid, or control pain. The physiologic component refers to nociception and the stress response. Pain can be acute or chronic, with different sensations related to the origin of the pain. Acute pain has a short duration, and it usually corresponds to the healing process (30 days), but should not exceed 6 months. It implies tissue damage that is usually from an identifiable cause. If undertreated, acute pain may bring a prolonged stress response and lead to permanent damage to the patient’s nervous system. In such instance, acute pain can become chronic.8,9 Chronic pain persists for more than 6 months after the healing process from the original injury, and it may or may not be associated with an illness.22 It develops when the healing process is incomplete or, as described earlier, when acute pain is poorly managed.23 Both acute and chronic pain can have a nociceptive or neuropathic origin.11 Nociceptive pain arises from activation of nociceptors,11 and it can be somatic or visceral. Somatic pain involves superficial tissues, such as the skin, muscles, joints, and bones. Its location is well defined. Visceral pain involves organs such as the heart, stomach, and liver. Its location is diffuse, and it can be referred to a different location in the body.24 Interestingly, not all organs are sensitive to pain and some can be damaged quite extensively without the patient feeling a thing. For instance, many diseases of the liver, the lungs or the kidneys are completely painless and the only symptoms felt are those derived from the abnormal functioning of these organs. On the other hand, relatively minor lesions in viscera such as the stomach, the bladder, or the ureters can produce excruciating pain, as these organs are abundantly innervated by sensory neurons that signal harmful events.25 Neuropathic pain arises from a lesion or disease affecting the somatosensory system.11 The origin of neuropathic pain may be peripheral or central. Neuralgia and neuropathy are examples related to peripheral neuropathic pain, which implies a damage of the peripheral somatosensory system. Central neuropathic pain involves the central somatosensory cortex and can be experienced by patients after a cerebral stroke. Neuropathic pain can be difficult to manage and frequently requires a multimodal approach (i.e., the combinations of several pharmacologic and/or nonpharmacologic treatments).24 Nociception represents the neural processes of encoding and processing noxious stimuli necessary, but not sufficient, for pain.11 Pain results from the integration of the pain-related signal into specific cortical areas of the brain associated with higher mental processes and consciousness. In other words, pain is the conscious experience that emerges from nociception.26 Four processes are involved in nociception24: The four processes are shown in Figure 9-1, which integrates pain assessment with nociception, and in Figure 9-2. As a result of transduction, an action potential is produced and is transmitted by nociceptive nerve fibers in the spinal cord that reach higher centers of the brain. This is called transmission, and it represents the second process of nociception. The principal nociceptive fibers are the A-delta (Aδ) and C fibers. Large-diameter, myelinated Aδ fibers that transmit well-localized, sharp pain are involved in “first pain” sensation, which leads to reflex withdrawal. Small-diameter, unmyelinated C fibers transmit diffuse, dull, aching pain, which is referred to as “second pain.”26 These fibers transmit the noxious sensation from the periphery through the dorsal root of the spinal cord. With the liberation of substance P, these fibers then synapse with ascending spinothalamic fibers to the central nervous system (CNS). These spinothalamic fibers are clustered into two specific pathways: neospinothalamic (NS) and paleospinothalamic (PS) pathways. Generally, the Aδ fibers transmit the pain sensation to the brain within the NS pathway, and the C fibers use the PS pathway.27 Through synapsing of nociceptive fibers with motor fibers in the spinal cord, muscle rigidity can appear because of a reflex activity.28 Muscle rigidity can be a behavioral indicator associated with pain. It can contribute to immobility and decrease diaphragmatic excursion. This can lead to hypoventilation and hypoxemia. Hypoxemia can be detected by a pulse oximeter (Spo2) and by oxygen arterial pressure (Pao2) monitoring. A ventilated patient’s interaction with the machine (e.g., activation of alarms, fighting the ventilator) also may indicate the presence of pain.29 The pain message is transmitted by the spinothalamic pathways to centers in the brain, where it is perceived. Pain sensation transmitted by the NS pathway reaches the thalamus, and the pain sensation transmitted by the PS pathway reaches brainstem, hypothalamus, and thalamus.27 These parts of the CNS contribute to the initial perception of pain. Projections to the limbic system and the frontal cortex allow expression of the affective component of pain.30 Projections to the sensory cortex located in the parietal lobe allow the patient to describe the sensory characteristics of his or her pain, such as location, intensity, and quality.30,31 The cognitive component of pain involves many parts of the cerebral cortex and is complex. These three components (affective, sensory, and cognitive) represent the subjective interpretation of pain. Parallel to this subjective process, certain facial expressions and body movements are behavioral indicators of pain occurring as a result of pain fiber projections to the motor cortex in the frontal lobe. Modulation is a process by which noxious stimuli that travel from the nociceptive receptors to the CNS may be enhanced or inhibited. Pain can be modulated by ascending and descending mechanisms. A typical example of ascending pain modulation is rubbing an injury site, thus activating large A-beta (Aβ) fibers in the periphery. Stimulation of these fibers activates inhibitory interneurons in the dorsal horn of the spinal cord, effectively preventing nociceptive signal transmission from the periphery to the higher brain regions. The physiologic basis of this mechanism of pain modulation was elucidated by Melzack and Wall in 196532 and refers to the Gate Control Theory (GCT). Analgesia may also be produced at the level of the spinal cord and the brainstem (spinothalamic pathway) via the release of endogenous opioids and neurotransmitters. Endogenous opioids are naturally occurring morphine-like pentapeptides found throughout the nervous system and exist in three general classes: beta-endorphins, enkephalins, and dynorphins. These substances block neuronal activity related to nociceptive impulses by binding to opioid mu (µ) receptor sites in the central and peripheral nervous systems.27 In the ascending pain modulation mechanism, endogenous opioids may be produced in the brainstem, and the dorsal horn or exogenous opioids may be introduced by administration of an opioid analgesic. The released or introduced opioids bind to the mu-opioid receptors on nociceptive nerve fibers, blocking the release of substance P. In the descending pain modulation mechanism, the efferent spinothalamic nerve fibers that descend from the brain can inhibit the propagation of the pain signal by triggering the release of endogenous opioids in the brain stem and in the spinal cord. Serotonin and norepinephrine are important inhibitory neurotransmitters that act in the CNS. These substances are also released by the descending fibers of the descending spinothalamic pathway.33 The use of distraction, relaxation and imagery techniques can facilitate the release of endogenous opioids, and has been shown to reduce the overall pain experience.34 A biologic stress response is activated by pain, an obvious stressor.29 This stress response involves the nervous, endocrine, and immune systems in the hypothalamic-pituitary-adrenal axis (HPA).35 The biologic stress response includes a short-term direct response, a midterm response, and a long-term indirect response. Stress mechanisms are depicted in Figure 9-3. In the presence of a stressor such as pain, the hypothalamus releases corticotropin-releasing factor (CRF), which activates the sympathetic nervous system (SNS). Norepinephrine is then released from the terminals of sympathetic nerves, and epinephrine is released from the adrenal cortex. This mechanism constitutes the short-term direct stress response. The effects of these stress hormones allow observation of physiologic responses associated with activation of SNS. For instance, increased blood pressure, increased heart rate, and increased respiratory rate are common signs of acute pain.15,29,36 Moreover, pupil dilation can be observed.37 If pain persists over time or injuries are located in the bladder or the intestines, the parasympathetic nervous system (PNS) may be dominant. The blood pressure and heart rate may decrease rather than increase. The absence of pain-related indicators related to the activation of the SNS does not necessarily imply an absence of pain sensation.38 Long term, the stress hormones, specifically cortisol, influence the immune system in two ways: immunosuppression and release of cytokines.39 Cytokines may prolong by retroactivation the release of cortisol, which may exacerbate tissue damage, contributing to the chronic pain process.23 In summary, the biologic stress response allows observation of fluctuations in physiologic signs that represent a source of stress and may be associated with acute pain. The short-term signs are mainly related to SNS activation. Other signs, such as decreased diuresis and increased CVP and PAOP, relate to the midterm indirect stress response. The immune system is involved in the long-term indirect response of stress. No acute pain indicators have been associated with this process. All the indicators identified within the biologic stress response are not specific to pain because they can be attributed to other distress conditions, homeostatic changes, and medications.16 As previously stated, self-report of pain is not always possible to obtain in critically ill patients as many of them may be unable to communicate during their stay in the critical care unit. When the patient is unable to self-report, the expression of pain can be examined from the perspective of the Communications Model of Pain.40 The foundation of this model is that observational measures capture behaviors that are less subject to voluntary control and more automatic in comparison with self-report measures that depend on higher mental processes. Consequently, observational measures should be used to assess pain when the individual’s self-report is not available, as is often the case in critically ill patients. This A → B → C model conceptualizes pain as an internal state (A) that may be encoded in particular features of expressive behaviors (B), allowing observers (in this case nurses) to draw inferences (C) about the nature of the sender’s experience (Fig. 9-4). Pain is known as a subjective experience. The subjective component of pain assessment refers to the patient’s self-report about his or her sensorial, affective, and cognitive experience of pain. Because it is considered the most valid measure of pain, the patient’s self-report must be obtained whenever possible.16 A simple yes or no (presence versus absence of pain) is a valid self-report. Mechanical ventilation should not be a barrier for nurses to document patients’ self-reports of pain. Many mechanically ventilated patients can communicate that they have pain or can use pain scales by pointing to numbers or symbols on the scale.2,4,15 Attempts should be made before concluding that a patient is unable to self-report. More importantly, sufficient time should be allowed for the patient to respond with each attempt.16 If sedation and cognition levels allow the patient to give more information about pain, a multidimensional assessment can be documented. Multidimensional pain assessment tools including the sensorial, emotional, and cognitive components are available, such as the Brief Pain Inventory,41 the Initial Pain Assessment Tool,24 and the McGill Pain Questionnaire–Short Form.42 However, because of the administration of sedative and analgesic agents in mechanically ventilated patients, the tool must be short enough to be completed. For instance, the McGill Pain Questionnaire–Short Form takes 2 to 3 minutes to complete and has been used to assess mechanically ventilated patients who were in a stable condition.2 The patient’s self-report of pain can also be obtained by questioning the patient using the mnemonic PQRSTU43: The Q in the mnemonic refers to the quality of the pain or the pain sensation that the patient is experiencing. For instance, the patient may describe the pain as dull, aching, sharp, burning, or stabbing. This information provides the nurse with data regarding the type of pain the patient is experiencing (e.g., somatic or visceral). The differentiation between types of pain may contribute to the determination of cause and management. A patient who has had open-heart surgery may complain of chest pain that is shooting or burning.4 This information can lead the nurse to investigate for cutaneous or bone injuries as a result of a sternotomy. Another patient may describe a sharp thoracic pain that may lead the nurse to consider visceral pain as a result of pulmonary embolism. A verbal description of pain is important because it provides a baseline account, allowing the critical care nurse to monitor changes in the type of pain, which may indicate a change in the underlying pathology. R usually is easy for the patient to identify, although visceral pain is more difficult for the patient to localize.24 If the patient has difficulty naming the location or is mechanically ventilated, ask the patient to point to the location on himself or herself or on a simple anatomic drawing.44 S, the severity or intensity of pain, is a measurement that has undergone much investigation. Many pain intensity scales are available, including the descriptive and numeric pain rating scales that are often used in the critical care environment (Fig. 9-5). Many critical care units use a specific pain intensity scale. The use of a single tool provides consistency of assessment and documentation. Employment of a pain intensity scale is useful in the critical care environment. Asking the patient to grade his or her pain on a scale of 0 to 10 is a consistent method and aids the nurse in objectifying the subjective nature of the patient’s pain. However, the patient’s tool preference should be considered. When the patient’s self-report is impossible to obtain, nurses can rely on the observation of behavioral indicators, which are strongly emphasized in clinical recommendations and guidelines for pain management in nonverbal patients.16,17 Fluctuations in vital signs should never be used alone but rather considered as a cue to begin further assessment for pain. Pain-related behaviors have been described in critically ill patients and were also studied in the AACN Thunder Project II.45 Patients who experienced pain during nociceptive procedures were three times more likely to have increased behavioral responses such as facial expressions, muscle rigidity, and vocalization than patients without pain. Similar observations were found in a study of 257 mechanically ventilated critically ill adults.30 Patients who experienced pain during turning showed significantly more intense facial expressions (e.g., grimacing), muscle rigidity, and less compliance with the ventilator (e.g., fighting the ventilator) compared with patients without pain. Behavioral indicators are strongly recommended for pain assessment in nonverbal patients,16 and several tools have been developed and tested in critically ill adults including the Behavioral Pain Scale (BPS),46 the Critical-Care Pain Observation Tool (CPOT),47 the NonVerbal Pain Scale (NVPS),48 the Pain Behavioral Assessment Tool (PBAT),46 and the Pain Assessment and Intervention Notation (PAIN) algorithm.39 The BPS and the CPOT are supported by experts in critical care49,50 and are suggested for use in medical, postoperative, and non-brain trauma critically ill adults unable to self-report in the clinical guidelines of the Society of Critical Care Medicine (SCCM).17 Moreover, their implementation in critical care units has led to enhanced nursing practices of pain assessment and management,51 and improved patient outcomes including shorter durations of mechanical ventilation and stay in the critical care unit.52 The BPS shown in Table 9-1 was tested mostly in nonverbal mechanically ventilated patients with altered levels of consciousness.46,53–56 Its validity was supported with significantly higher BPS scores during nociceptive procedures (e.g., turning, endotracheal suctioning, peripheral venous cannulation) compared with rest or nonnociceptive procedures (e.g., arterial catheter dressing change, compression stocking applications, eye care). The authors of the BPS determined a cut-off score greater than 5 for the presence of pain. A positive association was found between nurses’ BPS ratings and conscious sedated patients’ self-report of pain intensity during turning.54 Good interrater reliability of BPS scores between several raters including nurses was reached. The BPS can be used quickly (2 to 5 minutes), and most clinicians were satisfied with its ease of use.46 However, some expressed concerns about the lack of conceptual clarity of certain items.49 For instance, scores of 3 (i.e., fighting ventilator) and 4 (i.e., unable to control ventilation) for compliance with the ventilator category may be ambiguous. Similarly, movements with upper limbs category may be confused with muscle tension. TABLE 9-1 From Payen JF, et al. Assessing pain in the critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29(12):2258. The CPOT shown in Table 9-2 was tested in verbal and nonverbal, critically ill adult patients.15,29,47,57 Content validity was supported by critical care unit expert clinicians, including nurses and physicians.58 Validity of the CPOT was supported with significantly higher CPOT scores during a nociceptive procedure (e.g., turning with or without other care) compared with rest or a nonnociceptive procedure (e.g., taking blood pressure). Positive associations were found between the CPOT scores and the patient’s self-report of pain.15,29,47 A cut-off score greater than 2 was established with the CPOT in postoperative critical care unit adults.59 Similarly to the BPS, good interrater reliability of CPOT scores was achieved with critical care nurses.15,47 Feasibility and clinical utility of the CPOT were positively evaluated by critical care nurses.60 Nurses agreed that the CPOT was quick enough to be used in the critical care unit, simple to understand, easy to complete, and helpful for nursing practice. An online teaching video to learn how to use the CPOT at the bedside is available (see Table 9-2). TABLE 9-2 CRITICAL CARE PAIN OBSERVATION TOOL (CPOT) 1. The patient must be observed at rest for one minute to obtain a baseline value of the CPOT. 2. Then the patient should be observed during painful procedures (e.g., turning, wound care) to detect any changes in the patient’s behaviors. 3. The patient should be evaluated before and at the peak effect of an analgesic agent to assess whether the treatment was effective or not in relieving pain. 4. The patient should be attributed the highest score observed during the observation period. 5. The patient should be attributed a score for each behavior included in the CPOT and muscle tension should be evaluated last as it may lead to behavioral reactions not necessarily related to pain, but more to the actual stimulation. According to compliance with the ventilator, the nurse must check that the endotracheal tube is well positioned, and for the presence of secretions which could lead to higher scores for this item. An online teaching video funded and created by Kaiser Permanente Northern California Nursing Research (KPNCNR) to learn how to use the CPOT at the bedside is available at http://pointers.audiovideoweb.com/stcasx/il83win10115/CPOT2011-WMV.wmv/play.asx. Modified from Gélinas C, et al. Validation of the Critical-Care Pain Observation Tool (CPOT) in adult patients. Am J Crit Care. 2006;15:420. Figure of facial expressions a courtesy of Caroline Arbour, RN, BSc, PhD candidate, McGill University, Canada, and redrawn by Elsevier. A cut-off score refers to the score on a specific scale associated with the best probability of correctly ruling in or ruling out a patient with a specific condition—in this case pain. The use of a cut-off score with behavioral pain scales can help to identify when pain is highly likely to be present, and guide nurses in determining whether an intervention to alleviate pain is required or not. Also, a cut-off score can help to evaluate the effectiveness of pain-management interventions. It is important to highlight that cut-off scores are established using a criterion (i.e., a gold standard in the field). As mentioned previously, in the case of pain, the patient’s self-report is known as the gold standard criterion.11 For a case example showing how a cut-off score can be used in practice, refer to Box 9-1. Behaviors have been validated for pain assessment in critically ill patients, but they present some limitations. In fact, they are impossible to monitor in patients unable to respond behaviorally to pain such as those suffering from paralysis or under the effects of neuromuscular blocking agents. Also, behavioral responses may be blurred with the administration of high doses of sedative agents.15 Indeed, minimal behavioral responses to painful procedures were found in unconscious, mechanically ventilated critically ill adults who were more heavily sedated compared with conscious patients.29 Similar results were found in previous studies in which patients who received a higher dose of midazolam obtained a lower score on the BPS.56 In addition, behavioral pain scales developed for nonverbal critically ill patients may not be applicable for those with a brain injury and an altered level of consciousness as they were found to exhibit atypical behavioral responses to pain.29,61,62 Instead of frowning and grimacing, brain-injured patients with altered levels of consciousness seemed to react mostly by opening their eyes, showing tears, opening their mouth, and exhibiting repetitive movements of the lower limbs when exposed to pain. Further studies are needed to better understand how brain-injured patients react to painful procedures. Therefore existing behavioral pain scales may be inappropriate for this specific vulnerable group. When selecting a scale, nurses should make sure that is has been tested in a patient population and context in which they plan to use it. Indeed, a scale can only be shown to be valid with a specific group of people and in a given context.63 When patients cannot react behaviorally to pain, the only possible clues left for the detection of pain are physiologic indicators (i.e., vital signs). Although vital sign values generally increase during painful procedures,15,29,36,46,56 they are not consistently related to the patient’s self-report of pain, nor are they predictive of pain.15,29 For example, none of the monitored vital signs (heart rate, mean arterial pressure [MAP], respiratory rate, transcutaneous oxygen saturation [Spo2], and end-tidal CO2) predicted the presence of pain in critically ill patients.29 In the ASPMN recommendations and the SCCM guidelines, it is stated that vital signs should not be considered as primary indicators of pain because they can be attributed to other distress conditions, homeostatic changes, and medications. Changes in vital signs should instead be considered a cue to begin further assessment of pain or other stressors.16,17 Physiologic measures other than vital signs can support the nurses in detecting the presence of pain in nonverbal critically ill patients especially when behavioral indicators are no longer available. Other than vital signs, human brain reactivity has been studied using brain imaging technology such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) in healthy individuals and in patients with clinical pain conditions.64 Many regions of the brain are involved in the perception of pain, including the somatosensory cortex, the frontal cortex, and the thalamus. The anatomic connections between these regions suggest that they function in an interactive way in encoding the different aspects of pain (sensory and affective components of pain). For instance, the somatosensory cortex plays a major role in processing the sensory component of pain, whereas the frontal cortex appears to reflect the affective component of pain.26 A closer look into brain activity may elucidate how pain inputs are first received and processed within the cerebral cortex, offering a direct and more precise indicator of pain.
Pain and Pain Management
Importance of Pain Assessment
Definition and Description of Pain
Components of Pain
Types of Pain
Acute Pain
Chronic Pain
Nociceptive Pain
Neuropathic Pain
Physiology of Pain
Nociception
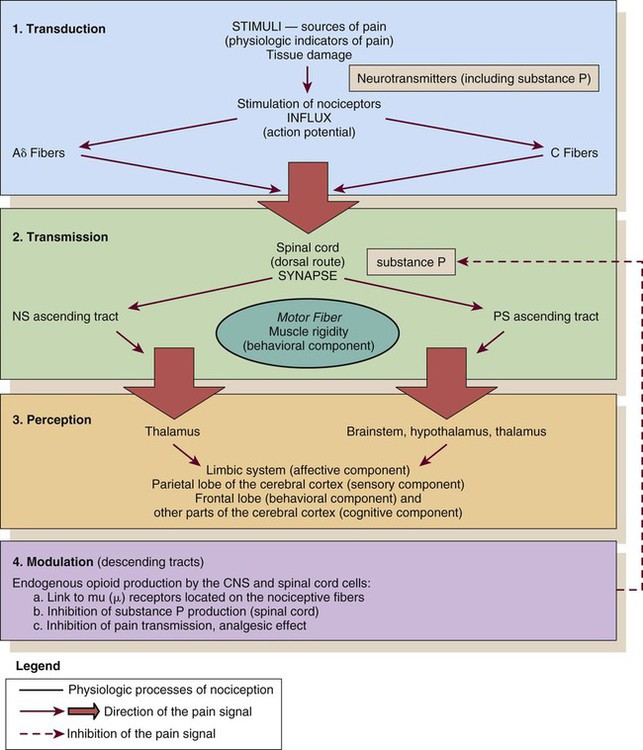
CNS, central nervous system; NS, neospinothalamic pathway; PS, paleospinothalamic pathway. (Courtesy Céline Gélinas, Ingram School of Nursing, McGill University, Canada.)
Transmission
Perception
Modulation
Biologic Stress Response
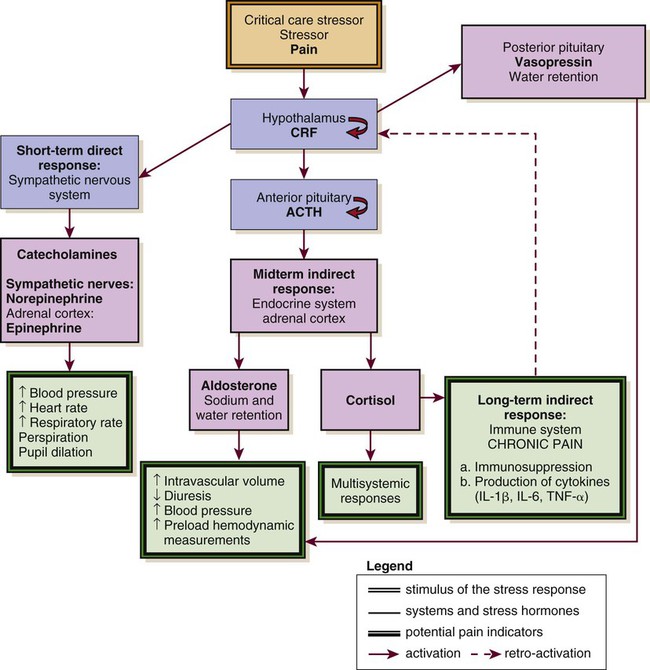
ACTH, adrenocorticotropic hormone; CRF, corticotropin-releasing factor; IL, interleukin; TNS, tumor necrosis factor. (Courtesy Céline Gélinas, Ingram School of Nursing, McGill University, Canada.)
Short-Term Direct Response
Long-Term Indirect Response
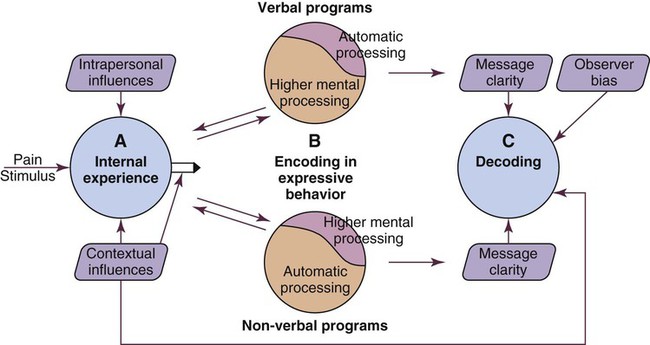
Framework for Pain Assessment and Definition
Pain Assessment
Pain Assessment: The Subjective Component
Q: Quality
R: Region or Location, Radiation
S: Severity and Other Symptoms
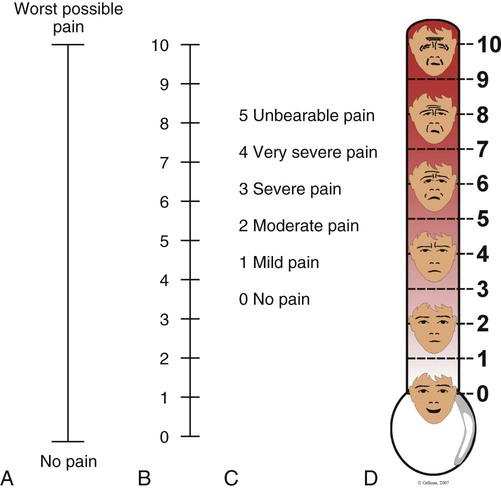
A, Visual analog scale (VAS). B, Numeric Rating Scale (NRS). C, Descriptive Rating Scale (DRS). D, Faces Pain Thermometer. (Courtesy Céline Gélinas, Ingram School of Nursing, McGill University, Canada.)
Pain Assessment: The Observable or Objective Component
Behavioral Pain Scale
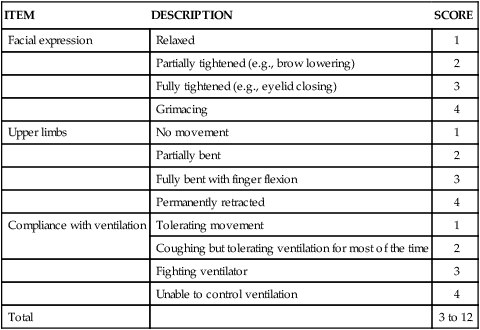
ITEM
DESCRIPTION
SCORE
Facial expression
Relaxed
1
Partially tightened (e.g., brow lowering)
2
Fully tightened (e.g., eyelid closing)
3
Grimacing
4
Upper limbs
No movement
1
Partially bent
2
Fully bent with finger flexion
3
Permanently retracted
4
Compliance with ventilation
Tolerating movement
1
Coughing but tolerating ventilation for most of the time
2
Fighting ventilator
3
Unable to control ventilation
4
Total
3 to 12
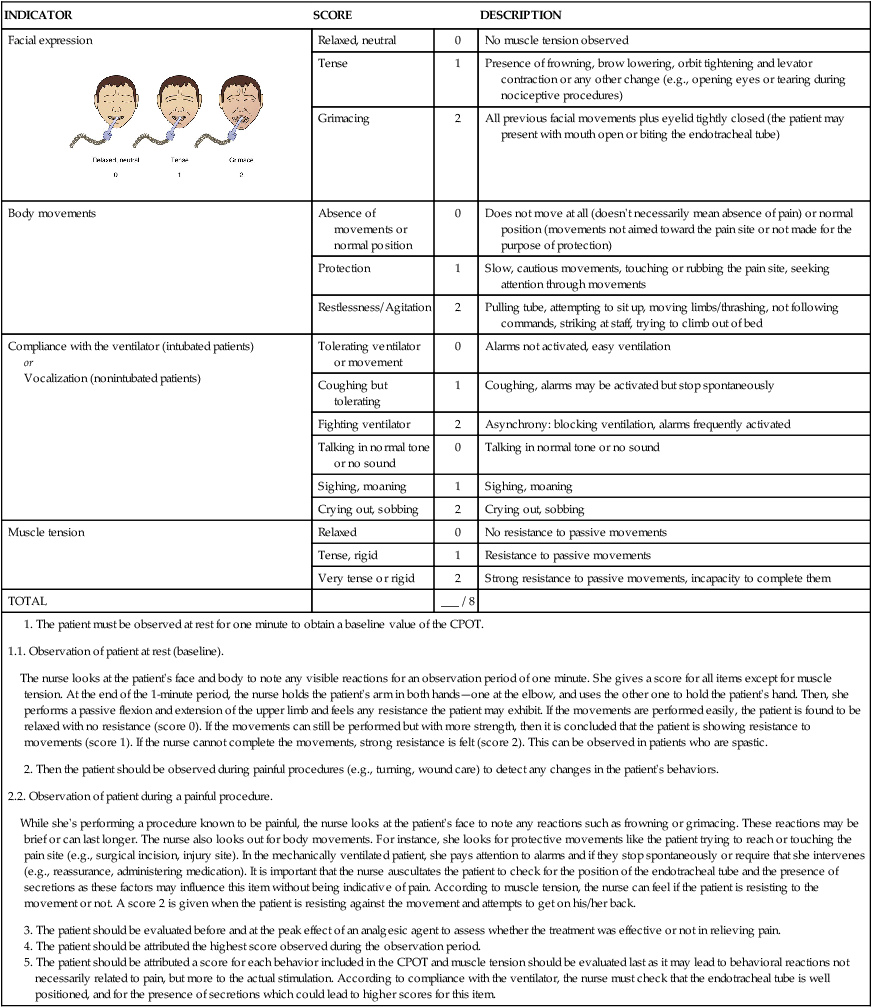
Critical-Care Pain Observation Tool
INDICATOR
SCORE
DESCRIPTION
Facial expression
Relaxed, neutral
0
No muscle tension observed
Tense
1
Presence of frowning, brow lowering, orbit tightening and levator contraction or any other change (e.g., opening eyes or tearing during nociceptive procedures)
Grimacing
2
All previous facial movements plus eyelid tightly closed (the patient may present with mouth open or biting the endotracheal tube)
Body movements
Absence of movements or normal position
0
Does not move at all (doesn’t necessarily mean absence of pain) or normal position (movements not aimed toward the pain site or not made for the purpose of protection)
Protection
1
Slow, cautious movements, touching or rubbing the pain site, seeking attention through movements
Restlessness/Agitation
2
Pulling tube, attempting to sit up, moving limbs/thrashing, not following commands, striking at staff, trying to climb out of bed
Compliance with the ventilator (intubated patients)
or
Vocalization (nonintubated patients)
Tolerating ventilator or movement
0
Alarms not activated, easy ventilation
Coughing but tolerating
1
Coughing, alarms may be activated but stop spontaneously
Fighting ventilator
2
Asynchrony: blocking ventilation, alarms frequently activated
Talking in normal tone or no sound
0
Talking in normal tone or no sound
Sighing, moaning
1
Sighing, moaning
Crying out, sobbing
2
Crying out, sobbing
Muscle tension
Relaxed
0
No resistance to passive movements
Tense, rigid
1
Resistance to passive movements
Very tense or rigid
2
Strong resistance to passive movements, incapacity to complete them
TOTAL
___ / 8
Use of Cut-Off Scores
Limitations Related to the Use of Behavioral Pain Scales
Physiologic Indicators
Cerebral Monitoring and Pain Assessment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pain and Pain Management
Get Clinical Tree app for offline access