The evolution of the immune system
Overview of the immune system
Innate immunity
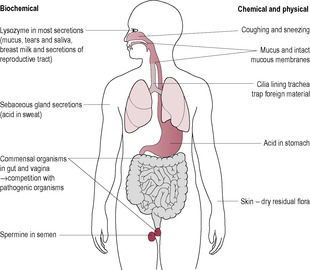
Fig. 10.2
Phagocytosis
Neutrophils and macrophages adhere to the surface of the target organism. Adherence is enhanced by opsonins, which form a bridge between the pathogen and the phagocyte. The phagocytic cells produce pseudopodia facilitating the engulfing of the pathogen into a cellular vesicle. Lysosomes fuse with the phagosome and degrade it
Cytotoxicity
Eosinophils and NK cells adhere to targets (opsonins increase efficiency). Eosinophils secrete chemicals, which damage the target cell membrane, causing cell death, and an inflammatory response, which is particularly effective against parasites. NK cells attack body cells expressing viral proteins in their membranes and some tumour cells. The NK cells adhere and release perforin, which penetrates the cell membrane causing cell death
Inflammation
The sequence of events is that the trigger (such as a bacteria signal) stimulates vasodilation and increased blood flow and delivery of blood cells (redness, heat and pain). Vascular permeability (swelling) occurs, which increases exudation and extracellular fluid (oedema), phagocyte invasion, promotion of fibrin wall enclosing infection and tissue repair
Lysozyme
The complement system
Interferons
Leukocytes and lymphocytes
Phagocytes
Natural killer cells
Acquired or specific immunity
Antigen recognition
Clonal selection and immunological memory
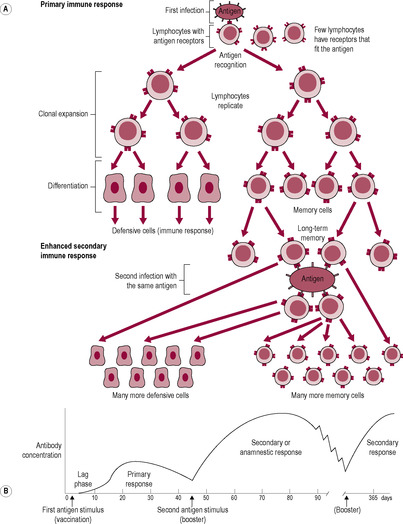
Fig. 10.3
Active Natural
Artificial
Passive Natural
Artificial
Clinical or subclinical disease
Vaccines: dead or extract attenuated toxoids
Congenital (across placenta) colostrum
Antiserum antitoxin gamma globulin
Passive immunity
Lymphocytes
B lymphocytes
Antibody
Role and Characteristics
IgG
Most abundant antibody (85% circulating antibody), found in blood and all fluid compartments including cerebrospinal fluid. Produced in large amounts at secondary adaptive response, therefore represent ‘history’ of past exposure to pathogens. Long lasting. Can diffuse out of bloodstream to site of acute infection and can cross placenta. Act as powerful opsonins bridging phagocyte and target cell. Important in defence against bacteria and activation of the complement system via the classic pathway
IgM
IgM molecules join in groups of five ‘IgM pentamers’, therefore tend to aggregate antigens into a clump that is a target for phagocytes and NK cells. Large molecules so cannot diffuse out of bloodstream. Very powerful activators of complement, important in immune responses to bacteria. First antibody produced when the body is confronted by a new antigen
IgA
Mostly in secretions such as saliva, tears, sweat and breast milk, especially colostrum. Link in groups of two to three. Protects body by adhering to pathogen and preventing its adherence to body cavity. Cannot activate complement or cross placenta
IgE
Tail binds to receptor on mast cells so involved in acute inflammation, allergic responses and hypersensitivity. Binding sites for antigens on larger parasites such as worms and flukes. Some people have IgE for common harmless environmental proteins such as pollen, fur, house dust mite and penicillin
IgD
Rarely synthesized; little is known about its functions. Large, found only in blood. May be involved in antigen stimulation of B cells
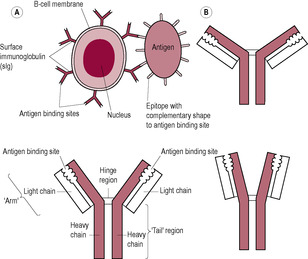
Fig. 10.4
T lymphocytes
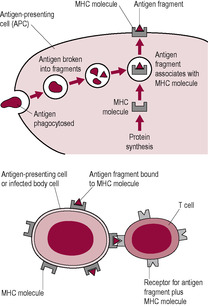
Fig. 10.5
Interaction of B and T lymphocytes
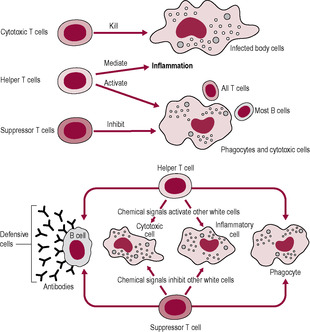
Fig. 10.7
Interaction of the innate and adaptive immune systems
The immune system in pregnancy; acceptance of the fetus
Fetal antigen expression
The uterus as a privileged site
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Overview of immunology
Zara and James try and live a healthy lifestyle; they both have never smoked and have not drunk any alcohol for many years. They follow a vegetarian diet and prefer to have organic, fresh products that are unprocessed and rely on milk products, nuts and pulses as the main source of protein in their diet. What precautions do Zara and Steve need to take and what particular types of food do they need to avoid and what are the reasons for this?
As part of their preparation to work in Africa, both Zara and James underwent in-depth medical screening to ensure that they were fit enough to undertake this work. Zara has the blood group O Rhesus negative and James has the blood group AB negative.
• As they are both Rhesus negative, then Rhesus incompatibility is not going to occur, but what possible complications could arise in the pregnancy as a result of these different blood groups, how could they be recognized and what are the possible treatments that may be required?
• What advice would women need before, during and after pregnancy in regard to travelling to or from tropical countries?
The immune system is a complex network of specialized cells and chemical signals, which interact to provide a defence against infectious organisms. A number of microorganisms are associated with the body. Some, described as commensal organisms, exist within or on their host without causing any harm. Others are beneficial, or symbiotic, like those inhabiting the skin and the gut. However, some organisms, including microbes (bacteria, viruses, fungi, etc.), and larger organisms like tapeworms, are potentially damaging and are described as pathogens because they cause disease. The immune system of man has evolved to detect and eradicate these pathogenic organisms. Pathogens are diverse and numerous and have a rapid rate of replication and therefore are constantly evolving their own mechanisms to combat the host’s defence.
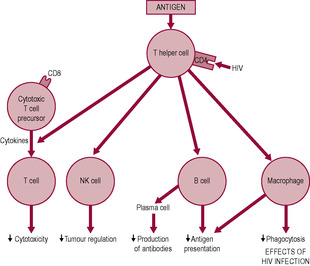
The role of the immune system becomes clearly evident when it is compromised such as in acquired immune deficiency syndrome (AIDS) when HIV causes breakdown of the immune system (Fig. 10.1). However, under less extreme conditions, both infections and poor nutrition can overwhelm the immune system. To some extent, both the pregnant woman and the neonate are immunocompromised, but the concept that pregnancy is a state of immunosuppression which increases susceptibility to infectious diseases is controversial. It does not make evolutionary sense for pregnancy to be dangerous from an immunological perspective. Reproduction is crucial for survival of the species; a paramount aspect of this is that the immune system, and therefore the survival of the mother and fetus, is strengthened by enhanced recognition, cell communication, trafficking and repair mechanisms (Mor and Cardenas, 2010). It is preferable to describe pregnancy as a state of immunomodulation. An alternative explanation to the apparent increased risk of infection in pregnancy is that the mother is more readily sensitized by the changes that occur in her immune system.
The detection of the presence of pathogens initiates the immune response in the host, stimulating a cascade of interactions, which culminates in a counter-attack on the pathogen. There are two types of immunity: innate immunity and adaptive immunity.
Innate (natural) immunity pre-exists in an organism before any contact with pathogens; it is a collection of genetically encoded responses to foreign pathogens which does not change throughout the lifespan. Innate immunity occurs throughout the plant and animal kingdom, occurring in mammals, birds, sponges and worms. It evolved early and is particularly effective against bacteria, probably the earliest form of life on earth. It mounts an immediate non-specific response to an invading microorganism.
The second type of immunity, adaptive or acquired immunity, is facilitated by mechanisms that adapt to the presence of pathogens and becomes more effective with each exposure. As organisms became more complex and colonized new habitats, they were vulnerable to a broader range of more recently evolved pathogens such as viruses. Adaptive immunity occurs exclusively in higher multicellular organisms that have evolved relatively recently, such as mammals, birds and some fish (jawed vertebrates). It has evolved in response to increased pressure on survival and augments innate immunity; it is specific and effective at eliminating infection. The adaptive immune system ensures that, if an animal survives an initial infection by a pathogen, it is usually immune to further illness caused by the same pathogen; this response is exploited in medical vaccination programmes. Although the innate and adaptive systems operate differently, there are many common mechanisms and components. It has been hypothesized that the innate system, though more ‘primitive’, plays a critical role in viviparity and toleration of the fetal allograft (Sacks et al., 1999).
Innate immunity is inherent and does not require contact with a pathogen for responses to occur. The defence is non-specific but not long-lasting. The responses are mobilized quickly and activated by receptors that generically respond to a broad range of pathogens. The first line of defence can be considered to be the physical and chemical barriers of the respiratory, reproductive and gastrointestinal systems and skin (Brostoff et al., 1991; Fig. 10.2). The skin is an impermeable barrier which is naturally acidic and undergoes continual desquamation. The gastrointestinal and respiratory tracts utilize peristalsis and cilia, respectively, to keep potentially infectious moieties moving. Commensal organisms on the skin or the epithelium of the respiratory and gastrointestinal systems create an environment which is hostile to pathogens. In addition, various secretions such as sweat, tears and secretions in the respiratory and gastrointestinal systems contain antimicrobial substances. The chemical protective mechanisms of the innate immune system include that provided by lysozyme, the complement system, interferons and phagocytotic activity of the white blood cells (Table 10.1). The responses of the innate immune system, such as inflammation, generate cytokines (chemical signals) which recruit immune cells to the site of infection, resulting in identification and removal of dead cells and foreign substances and also communicate with the adaptive immune system via presentation of antigens.
(Reproduced with permission from Brostoff et al., 1991.)
Cells of the innate immune system express pattern recognition receptors (PRRs) which recognize pathogen-associated molecular patterns (PAMPs) which are unique molecular sequences expressed on the surface of pathogenic microorganisms (Koga et al., 2009). When PRRs of the immune cells bind to PAMPs on the pathogen, an inflammatory response is generated against the pathogen. One of the main families of PRRs is the Toll-like receptors (TLRs). Eleven types of TLRs have been identified in humans. TLRs are very specific to particular PAMPs that are molecules which are evolutionary conserved and critical to the pathogen’s function (Koga and Mor, 2010). For instance, TLR4 recognizes gram-negative bacterial lipopolysaccharide and TLR2 recognizes bacterial lipoproteins, gram-positive bacterial peptidoglycans and fungal zymosan. TLRs also act with host cells which display ‘danger signals’, for instance, express different molecules on their surface when they are apoptotic or stressed or damaged, for instance, by reactive oxygen species.
Lysozyme, which is sometimes described as ‘the body’s own antibiotic’, is an enzyme that attacks the unique polysaccharide structure of bacterial cell walls. It is an abundant component of body secretions such as blood, sweat, tears, nasal secretion, breast milk and the mucous secretions of the reproductive tract.
The complement system involves over 30 interacting proteins, mostly synthesized by the liver, and receptors which form an amplification cascade of defence, leading to cytolysis of bacteria, chemotaxis, opsonization and inflammation. As in the blood coagulation cascade, the components of the complement cascade exist in an inactive precursor form that can be triggered and activated. There are three pathways of activation: the classic pathway, which involves antibodies (or immunoglobulins), the alternative pathway, where the complement cascade is activated by the unique composition of an organism’s cell wall (activator surfaces), and the mannan-binding lectin pathway. The outcome of complement activation is the formation of a membrane attack complex, cylindrical assembly of proteins that form a tube that perforates the plasma membrane of target cells. This perforation allows ions to enter cells so that fluid follows by osmosis and the bacterial cells swell and burst. The complement cascade stimulates the release of histamine and kinins from mast cells, recruits macrophages and neutrophils to the site and enhances phagocytosis by opsonization. The complement system may be a factor in a number of diseases such as multiple sclerosis, asthma, Alzheimer’s disease, type 1 diabetes (Hewagama and Richardson, 2009) and autoimmune diseases such as SLE (Chen et al., 2010).
Interferons are cytokines secreted by virally infected cells that carry out a non-specific defence which prevents viral replication. Viruses replicate by ‘hijacking’ protein synthesis in the host cells they have infected. Therefore, the cell is diverted to make viral mRNA, which is translated into viral proteins and assembled as viral particles. Interferons interfere with this production of new viral proteins; they damage the viral mRNA and inhibit protein translation, not just in the infected cells but in neighbouring uninfected cells as well, subsequently creating a barrier around the viral infection, which prevents the viral replication. Interferons also stimulate macrophages and natural killer (NK) cells; they can also up-regulate major histocompatibility complexes (MHCs). Interferons produced by genetic engineering are used therapeutically.
The cells of the immune system are leukocytes and lymphocytes. Although they are described as white blood cells, some of the cells spend very little time in the circulation, whereas others never enter the vascular system at all and remain in the lymphatic system, spleen or other tissues. Blood cells are derived from a single population of haemopoietic stem cells (HSCs) in the bone marrow. These precursor cells have the potential to divide into progenitor cells which can differentiate into all the cell types present in blood. After radiation for cancer treatment, the destroyed stem cells have to be replaced by a bone marrow transplant; very few cells need to be transplanted for regeneration of a mature population of cells.
Neutrophils and macrophages are phagocytic white blood cells that can engulf and digest foreign cells and unwanted matter, such as the body’s own dead and dying cells, through the processes of phagocytosis, cytotoxicity and the generation of an inflammatory response. The cells that mediate innate immunity are the granulocytes: neutrophils, monocytes, eosinophils and basophils. Neutrophils, also known as polymorphonuclear leukocytes, are the most numerous, forming about 40–70% of the circulating white blood cells. On entering the circulation, neutrophils, which have a lifespan of a few days, cease cell division. Neutrophils are motile and exhibit chemotactic behaviour, moving through a concentration gradient towards chemical messengers, such as those released from dividing bacteria, activated platelets or other phagocytotic cells, towards the site of infection. Neutrophils have multilobed nuclei which aid diapedesis (the amoeboid movement of the cells through the gaps between the capillary endothelial cells). Neutrophil phagocytosis is fast. Neutrophils usually reach the site of infection and begin phagocytosis within about 90 min of the initial stimulation; they surround the unwanted material and engulf it in a phagosome which merges with a lysosome containing substances that kill the engulfed bacterium or neutrophil granules to form a phagolysosome. The neutrophil can generate a vigorous and lethal respiratory burst releasing lethal reactive oxygen species or produce nitric oxide which kills both the phagocyte itself and the engulfed pathogen. Alternately, the bacterium is killed by myeloperoxidase from neutrophil granules which can generate hypochlorite (bleach) which is very toxic to bacteria; the green haem pigment of myeloperoxidase gives the greenish colour of bacterially infected mucous and ‘pus’.
Adherence of the phagocyte to the target cell can be increased by opsonization. Opsonins include antibodies which bind specifically to an antigen on the pathogen surface and are then recognized by Fc receptors on phagocytes so that the pathogen is more efficiently recognized and phagocytosed. The complement component, C3, also binds to pathogens and is recognized by the complement receptor on phagocytes, thus increasing the efficiency of recognition and adherence of the phagocyte. Effectively, the opsonin acts as a bridge between the pathogen and the phagocyte, so promoting phagocytosis. Monocytes circulate for a short time in the bloodstream and then migrate to tissues and organs where they differentiate into macrophages and exhibit characteristics specific to their host tissue. Less than 7% of circulating white blood cells are monocytes, but ‘resident macrophages’ are abundantly distributed in the body tissues and are particularly dense around blood vessels, the gut walls, the genital tract and lungs. Macrophages are oestrogen-sensitive. Monocytes are the largest white blood cell and have a characteristic horse-shaped nucleus. They are also phagocytes and have a longer lifespan than neutrophils. Circulating monocytes respond more slowly than neutrophils, reaching the site of infection within about 48 h, but they have a greater capacity for phagocytosis, engulfing more material than neutrophils.
Many pathogens have evolved mechanisms to evade phagocyte activity; these include inhabiting niches like the skin where phagocytes cannot reach, suppressing inflammatory responses, interfering with the phagocyte recognition of pathogens or interfering with chemotaxis. In addition, some bacteria can block phagocytosis or survive within phagocytes or in the phagolysomes.
NK cells are cytotoxic lymphocytes which attack compromised host cells such as virally infected and cancerous cells. Although NK cells are lymphocytes, they are part of the innate immune system. NK cells kill cells which express low levels of MHC proteins, which is a common characteristic of both virally infected cell and some cancer cells. NK cells alter the target cell’s plasma membrane by releasing proteases and perforins from their cytoplasmic granules so water and ions diffuse in; consequently, the virus-infected cell swells and lyses or dies by apoptosis. To recognize the body’s own cells which are infected or behaving abnormally, NK cells have to be able to distinguish between self and altered-self state. Apoptotic cells are tagged by the phophatidylserine phospholipids of the cell membrane, which are usually orientated on the internal cytosolic face of the membrane, ‘scrambling’ to the exterior of the membrane which alerts phagocytotic cells.
Lymphocytes, which constitute about 20–40% of the circulating white blood cells, coordinate the adaptive immune responses. These small cells have relatively little cytoplasm, few organelles and no granules. T lymphocytes (or T cells) have secretory vesicles containing perforins and granzymes. B lymphocytes are dominant in humoral responses, mediated by immunoglobulins (antibodies) which attack bacteria and viruses in body fluids. T lymphocytes are dominant in cell-mediated immunity (see Chapter 1). Small lymphocytes also circulate in the lymphoid system and spend much of the time resident in the organs of the lymphoid system. The lymphoid system is the main site of the adaptive immune responses. Fluid leaks out of the blood capillaries into the intercellular spaces. Some of the fluid re-enters the blood capillary (see Chapter 1) but some enters the lymphatic capillaries. This lymph fluid, therefore, has a similar composition to plasma, except that the protein component of plasma is retained within the blood vessels so lymph fluid has low protein content. Ultimately, the lymph fluid is transported through lymph vessels to the thoracic duct and back into the bloodstream. The small lymphocytes ‘burrow out’ of the small veins as they pass through the lymph nodes and so enter the lymphoid tissue. Each lymphocyte spends minutes in the bloodstream compared with hours residing in the lymphoid system. Lymphocytes have different levels of maturity; naïve lymphocytes which are mature but have not encountered the antigen they will recognize, effector cells which have been activated by an antigen and memory cells which have survived from past exposure to the antigen and could rapidly divide in response to subsequent exposure to the antigen.
The classical view is that cells of the immune system differentiate between self (body) and non-self (foreign) cells (and changed self in host cells which are infected by a virus), identifying foreign cells and pathogens which have evaded the innate immune system and attack them. It has been hypothesized that the immune system differentiates between dangerous and non-dangerous rather than between self and non-self or infectious or non-infectious (Gallucci and Matzinger, 2001). The surface of a pathogen displays a unique combination of antigenic determinants that can be recognized by the immune cells as ‘foreign’ or non-self. The whole cell that engenders an immune response is described as an antigen, although the cluster of antigenic determinants itself is called the epitope. Each antigen can be displayed by the pathogenic cell itself, or can be secreted by a pathogen, for instance, bacterial toxins, or substances from non-pathogenic sources, such as plant pollens, resulting in allergic responses, or chemicals such as synthetic vaccines. Only certain parts of the entire antigen are immunogenic; these parts bind antibodies and activated lymphocytes. Most naturally occurring antigens have numerous antigenic determinants that can mobilize several different lymphocyte populations. Large chemically simple molecules (such as plastics) have little or no immunogenicity. Antigenicity depends on the ability of the host to identify the substance as an antigen; there are variations in individual responses.
Lymphocytes have high-affinity surface receptors that recognize antigens with very high specificity. Each lymphocyte has a single type of specific antigen receptor, unlike the cells of the innate (non-specific) immune system, which have many different types of receptor on each cell. These receptors differ between T lymphocytes (T-cell receptors) and B lymphocytes, the latter displaying on its cell surface copies of the specific antibody that each cell can secrete. Infective pathogens will normally display multiple antigens which are recognized as foreign by many different lymphocytes in the infected host. There are over 100 million pathogenic epitopes. Each person has, at birth, a population of lymphocytes consisting of clones, each of a few cells. A clone has a few identical lymphocytes, each of which has many copies of the same antigen receptor on its surface. The population of lymphocytes has the capability, described as its repertoire of receptors, to respond to a vast number of antigens, most of which are unlikely to be encountered in a lifetime. Initially, the number of cells expressing receptors for any particular antigen is small and this clone will remain small unless the antigen reacts. So, one of the first steps in mounting an effective immune response is to expand the clone by increasing the number of cells expressing the same antigen, a response termed ‘clonal selection’.
There are far more antigen receptors than there are human genes encoded for by DNA (about 100 million epitopes and only approximately 25000 different human genes). It is hypothesized, therefore, that each antigen receptor site is coded for by a few randomly selected genes. As there are several hundred possible genes involved, the random selection of a few genes that can be cut and spliced (somatic recombination) can produce enough combinations of antigen receptor gene segments to make all the 100 million epitope-binding sites. So, an individual can develop a huge number of diverse types of lymphocytes, each expressing a unique receptor for an antigen from a small family of genes. The gene segments recombine to form unique genes. This process is known as combinatorial diversification or V(D)J (variable, diverse and joining gene segment) recombination.
However, in the random production of antigen receptors, some lymphocytes will possess receptors for the body’s own antigens. In the fetal thymus, clonal deletion takes place, which results in the destruction or deletion of self- or autoreactive T lymphocytes by apoptosis. This process is called central tolerance. Any T lymphocyte binding to specialized cells presenting self-epitopes on the surface will be stimulated to undergo apoptosis. Self-reactive B lymphocytes do not need to be destroyed because they require a signal from a T lymphocyte (helper T cell) before they can function. It seems that some of the self-reactive T cells escape central tolerance in all individuals (Weetman, 2010). These are prevented from causing autoimmune disease in healthy people by a range of peripheral tolerance mechanisms (Mueller, 2010). It is important that the immune system has self-tolerance and does not harm cells or molecules of the host that are recognized as antigenic; otherwise, the host would be damaged (as happens in autoimmune diseases).
The immune response to a second and subsequent exposure to the antigen is faster and more effective than the first exposure (Fig. 10.3). The primary adaptive response, on the first exposure, is slow to develop, perhaps taking 7–14 days, and then builds slowly to a peak about 2 weeks later. The time for the response to become evident is termed the ‘incubation period’. Then, symptoms become apparent until the immune response has become effective. The secondary adaptive response, when the host subsequently encounters the same antigen, develops sooner, lasts longer and is more effective so signs of infection or symptoms may be prevented. On first exposure, the small numbers of lymphocytes binding the antigen are stimulated to undergo rapid cell division. A single lymphocyte can divide fast enough to produce 64000 daughter cells in 4 days. As the new cells, also bearing receptor sites specific to the stimulating antigen, are produced, they mature and differentiate. If B lymphocytes are activated, some members of the new population of lymphocytes are active in attacking the cells bearing the antigen. Most clone cells become antibody-secreting plasma cells. Others become memory cells, which have a long life and continue to circulate as a permanently enlarged clone of lymphocytes capable of recognizing specific antigens and mounting an immediate response. Effectively, the initial immune response is boosted by repeated exposure.
((A) Reproduced with permission from Stewart, 1997.)
Individuals have immunity to an antigen if their immune system can mount a fast and effective specific response to that antigen. The role of a vaccine is to deliberately stimulate the immune response and increase the clonal size without causing the illness. Effective vaccines can be in the form of killed whole organisms, harmless organisms, organisms that have been modified or attenuated (as in most viral vaccines), fragments of organisms (as in many bacterial vaccines), substances with similar epitopes, synthetic epitopes or inactivated toxins (Table 10.2). Some antigens are more effective at triggering clonal expansion, such as rubella (German measles) virus, which is highly antigenic; thus, after primary exposure, the host rarely acquires the infection again. Other pathogens, such as Neisseria gonococcus (causing gonorrhoea) and Treponema pallidum (causing syphilis), are only weakly antigenic; therefore, there are no effective vaccines available.
Resistance to a specific pathogen, acquired by previous exposure or deliberate immunization resulting in clonal expansion, is active immunity. However, sometimes, the effect of infection can be disastrous before the immune system has time to mount a response. Passive immunization can overcome this by providing temporary resistance in the form of products from a donor source. Passive immunization causes destruction of the pathogenic cells without creating clonal expansion or making memory cells so its effects are not permanent. An individual who has no prior immunity but is exposed to antigens or a potentially dangerous disease is given preformed antibodies. Examples include treatment following a bite from a rabid dog or anti-D immunization following potential exposure to Rhesus-incompatible antigens (see below). The transfer of placental antibodies (IgG) to the fetus and consumption of antibodies (IgA) in colostrum and breast milk by the neonate are also examples of passive immunity.
Case study 10.1 looks at an example of exposure to German measles.
Melanie is expecting her first baby. She attends the midwives’ clinic at 11 weeks’ gestation concerned over the welfare of her unborn baby. Her 3-year-old nephew, Michael, whom she sees regularly, has German measles.
• What factors would the midwife need to consider in advising Melanie over her concerns?
• Would there be any specific investigations to carry out?
If Melanie was susceptible to Rubella infection, how would this be recognized and what subsequent management and care be planned?
There are three types of lymphocytes: those that mature in the bone marrow, called B lymphocytes, those that mature in the thymus, called T lymphocytes and NK cells (see above).
B lymphocytes secrete antibodies which are responsible for the humoral immune response. On binding to an antigen, B lymphocytes undergo clonal expansion producing two types of daughter cells: memory B cells and plasma cells. The plasma cells are short-lived cells which synthesize and secrete large amounts of antibodies (immunoglobulins), which are specialized glycoproteins that bind specifically to the antigen that was recognized by the B lymphocyte. These antibodies bind to their target antigens and enable other components of the immune system, such as phagocytes and complement proteins, to attack the precise organism bearing the antigen rapidly and effectively. There are five classes of antibody (Table 10.3); these differ in the structure of the ‘Y’-shape of the tail, which affects whether the antibodies bind in groups or singly (Fig. 10.4). The antigen receptor on B lymphocytes is a surface immunoglobulin (sIg) closely resembling the structure of the antibody-binding site that will bind to the same antigen. The binding of the B cell to the epitope is relatively straightforward in that the antigen is intact or native, whereas T lymphocytes bind only to processed antigens. However, antigen binding by a B lymphocyte usually requires helper T-cell activity before clonal expansion can take place.
(Reproduced with permission from Stewart, 1997.)
There are three subsets of T lymphocytes: helper T cells, regulatory T (Treg) cells (formerly called suppressor cells) and cytotoxic T cells. Unique glycoproteins on the cell surface, which are involved in mediating cell function, can be identified using monoclonal antibodies and used to distinguish different subpopulations of T lymphocytes. The system of nomenclature is based on the cluster of differentiation (CD) system. Cytotoxic T cells express CD8 protein markers in the plasma membrane and recognize and destroy cells that have become infected or cancerous. Some regulatory T cells also express CD8 but express other markers as well, including CD4 and CD25, which distinguish them from other T-cell types.
T helper cells express CD4 proteins, and are sometimes called CD4 + cells. Helper T cells interact with macrophages and produce cytokines, which activate and regulate other components of the immune system. Cytokines are soluble polypeptides with a short range and lifespan that are also synthesized by lymphocytes and macrophages. They can induce fever, stimulate lymphocytes, stimulate antigen expression and potentiate the destruction of tumour cells. Cytokines include interleukins, interferons, tumour necrosis factor (TNF) and some colony-stimulating factors (CSF). Helper T cells induce proliferation of lymphocytes, stimulate antibody production by B lymphocytes and enhance the activity of cytotoxic T lymphocytes.
The receptor for HIV is the CD4 protein expressed not only by T cells but also by macrophages and possibly other cells. HIV reduces the number of helper T cells by inducing apoptosis so none of the immune mechanisms work effectively. Infection of macrophages shifts the profile of cytokines produced, which contributes to wasting and acute respiratory distress syndrome. Macrophages can act as a reservoir of the virus.
T lymphocytes have antigen receptors on their surface, formed of two peptide chains that contain the binding site for a specific epitope. However, the T lymphocyte cannot bind to an epitope unless it is has been processed and presented to the T lymphocyte by one of the host’s own cells. Although most nucleated cells have the capability of presenting an antigen and activating T lymphocytes, some cells generate a more efficient immunostimulatory response. These ‘professional’ antigen-presenting cells (APCs) are dendritic cells, B lymphocytes and macrophages that bind the antigen and phagocytose it, degrading its protein and then migrating to the lymph nodes. The resulting epitope fragments are displayed in the cleft of the MHC molecule (Box 10.1) on the surface of the APC (Fig. 10.5). Helper and Treg lymphocytes bind only to epitopes processed in this manner. Cytotoxic T cells recognize the epitopes of intercellular pathogens, such as viruses, that are incorporated into the cell membrane during cell replication of an infected cell harbouring the virus.
Box 10.1
Each person has a unique configuration of MHC antigens or molecules on the surface of their cells (except monozygous twins who have identical MHC). MHC molecules are a marker of ‘self’ and are synonymous with human leukocyte antigen (HLA). MHC molecules present the antigens to T cells. There are different classes of MHC molecules, which present antigens with different effectiveness. MHC molecules restrict helper T cells to interact with immune cells that have already bound to the epitope for which the T cell also has an antigen receptor. It is these cells that are involved in rejection of transplanted tissue, as all MHC molecules are different. Tissue grafts have increased survival if there is some similarity in MHC structure between the donor and the recipient (hence the need for tissue typing) and if drugs are used to suppress the immune response.
(Reproduced with permission from Stewart, 1997.)
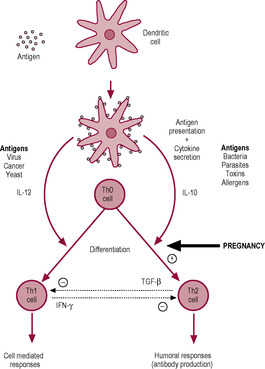
There are at least three forms of T helper cells: Th0, Th1 and Th2 cells, which are classified on the basis of their cytokine secretion. The dendritic cells present the antigen to T helper cells and direct them to differentiate into either Th1 or Th2 cells. When the resting T helper cells are initially activated, they become Th0 cells which have characteristics of both Th1 and Th2 cells; Th0 cells are then further activated and differentiate into either Th1 or Th2 cells depending on the type of threat. This pathway of differentiation is controlled by the cytokine secretion of the dendritic cell. Secretion of interleukins IL-12 and IL-10 by the dendritic cell drives the differentiation of Th1 and Th2 cells, respectively (Fig. 10.6).
The Th1 response tends to be initiated by viruses, cancer, yeasts and intracellular bacteria (e.g. Mycoplasma pneumoniae or Chlamydia). The outcome of the Th1 response is activation of cell-mediated immunity and the secretion of gamma-interferon (INF-γ), IL-2, lymphotoxin and granulocyte–macrophage colony-stimulating factor (GM-CSF). This activates cytotoxic T cells and NK cells to respond to target cells carrying intracellular pathogens or mutated proteins since these would not be responsive to circulating antibodies. Body cells continuously turn over their protein and some of these protein fragments or peptides are displayed on the cell surface in association with MHC proteins which the cells of the immune system monitor. Virally infected or cancerous cells display peptides from viral or mutated proteins which are recognized as foreign by the immune cells and displayed by APCs to provoke an immune response. Following clonal expansion of the T lymphocyte that recognizes the non-self peptide, cytotoxic T cells seek out the virally infected or mutated cells and, on contact, produce toxic and perforating substances that induce apoptosis and then turn the immune response off when the antigen-expressing cells have been eliminated When the infection has resolved, most of the cytotoxic T cells die and are removed by phagocytosis; a small percentage of the T cells remain as memory cells.
Other bacteria, parasites, toxins and allergens predominantly trigger a Th2 response and secretion of IL-3, IL-4, IL-5, IL-6, IL-10 and IL-13 which activate eosinophils, leukocytes and B lymphocytes (which produce antibodies). The Th1 and Th2 systems suppress each other; the Th1/Th2 balance is important. For instance, some viruses produce proteins that mimic IL-10 and drive the differentiation of Th0 cells into Th2 cells which are less effective at attacking viruses so viral survival is enhanced. In pregnancy, the Th1/Th2 balance shifts in favour of Th2; this downregulation of Th1-induced cellular immunity and enhancement of Th2-induced humoral responsiveness in the maternal immune system in pregnancy is mediated by progesterone and is important in preventing fetal rejection (see below).
Regulatory T cells limit the activity of the immune cells and prevent damage to the body’s own cells maintaining immune system homeostasis and tolerance to self-antigens (and are thus important in preventing autoimmune disorders) (Guerin et al., 2009). Treg cells are involved in maintaining peripheral tolerance. Treg cells accumulate in the lymph nodes draining the uterus and spleen in early pregnancy probably because they migrate towards human chorionic gonadotrophin (hCG) and chemokines produced by the trophoblast. Treg cells appear to be important in immune tolerance of the conceptus tissue (see below) and the sperm and oocytes.
B lymphocytes and T lymphocytes interact (Fig. 10.7). The B lymphocyte binds to the native (intact) antigen via the sIg receptors on its cell surface. The antigen is then internalized by the B lymphocyte and processed so fragments appear on its cell surface associated with the MHC molecule. In this form, the T lymphocytes can recognize it so helper T cells are activated, producing the signal that allows the B lymphocyte to start cell division and differentiation.
(Reproduced with permission from Stewart, 1997.)
The innate system is an integral part of the immune response. It initiates an immune response by the macrophages, processing an antigen in association with the MHC and presenting it to lymphocytes; this is called signal 1. The full response requires adjuvants, such as endotoxin, which produce signal 2, pro-inflammatory cytokines or co-stimulatory surface molecules. This signal conveys the biological significance of the antigen and effectively instructs the adaptive system to respond (or not). Signals 1 and 2 stimulate T cells to become effector Th1 or Th2 cells. Macrophages secrete cytokines which activate other macrophages, NK cells and granulocytes.
Humans are ‘outbred’. The genetic diversity resulting from sexual reproduction means that the fetus is phenotypically unique and immunologically distinct from both of its parents. The fetus has a unique combination of histocompatibility antigens. The fetus is classified as an allograft: foreign tissue from the same species but with different antigenic make-up. Half of the fetal antigens are derived from the father. There is a marked antigenic difference between the maternal tissues and the paternally inherited antigens expressed by the fetus. If tissue from the offspring is grafted on to its mother, a strong maternal immune response is mounted and the tissue is rejected. It seems surprising therefore that the mother does not reject the fetus because of its foreign antigens. Medawar (1953) proposed several possibilities to explain why the fetus is not rejected: as fetal tissue is antigenically immature, it may not express normal antigens, the uterus may be a privileged site (or not in contact with fetal tissue) or pregnancy may affect the maternal immune system and normal immune responses. However, it is the placenta and not the fetus that is the ‘transplant’, and the placenta has very different characteristics than that of a transplanted tissue or organ, and the interaction of the trophoblast with the maternal immune system orchestrates cooperative modulation of the components of the immune system.
Fetal tissue is antigenically mature and does express antigens and immunocompetence from an early stage (Koga and Mor, 2010). MHC class I and II antigens, albeit in smaller amounts, are present on embryonic cells from the time of implantation throughout the pregnancy. The MHC antigens are the molecules that are normally recognized by a transplant-recipient’s immune system and cause rejection of allografts (foreign tissue transplants), but trophoblast cells are an exception (Weetman, 2010). Paternal antigens are apparent at the eight-cell stage of the cleavage, and major histocompatibility antigens begin to be expressed at later stages of cell division. Dendritic cells from fetal skin are in contact with maternal blood and enter the maternal circulation; these cells express MHC antigens (Zenclussen et al., 2007). The zona pellucida and early trophoblast have glycoprotein coatings, which may limit the cell-mediated immune responses. Immunological problems are usually not a problem prior to implantation because the endometrium secretes immunosuppressive factors.
Both the mother and other individuals reject grafts of fetal tissue because fetal tissue expresses antigens. Maternal responses to transplanted tissue remain competent in pregnancy; a pregnant mammal rejects tissue from the father of the fetus and tissue from the fetus grafted to areas other than the uterus. Some tissues, such as the testis and parts of the eye and brain, lack components required in immune responses or are not accessible to them (Mellor and Munn, 2008). These sites are immunologically privileged and can accept transplanted tissue with fewer problems. However, the uterus is not a privileged site as was suggested (Billingham, 1964); the development of ectopic pregnancies shows that the uterus is not a uniquely immunoprivileged site. The increased vascularization of the pregnant uterus allows efficient delivery of lymphocytes and other maternal immune cells so non-fetal allogenic tissue transplanted in the uterus is rejected (Beer and Billingham, 1974). Maternal and placental tissue are closely situated and in close proximity. There are a number of interfaces between maternal immune cells and placental cells that change as the pregnancy progresses.
Get Clinical Tree app for offline access