Chapter 11 Overview of antiretroviral therapy
Introduction
The central goal of HIV therapy is maximal suppression of viral replication in order to allow immune recovery, and to prevent the selection of drug resistance mutations [1–3]. Successful ART must durably restore or maintain immune competence and the control of infections and malignancies that characterize the AIDS syndrome. Beyond that, ART should enhance and extend the healthy life span of treated individuals.
In the resource-rich world, the availability of newer ARV medications and new classes of ARVs has made it possible to achieve virologic suppression even in most patients infected with HIV resistant to multiple ARV agents or entire drug classes [4–6]. For those patients in whom therapy does not fully suppress viremia, ART can still dramatically slow disease progression [7, 8]. While ART does improve immune function and reduce morbidity and mortality associated with HIV (most strikingly in those with lower CD4 counts), control of HIV viremia unfortunately may not result in complete normalization of immune function or complete reversal of the inflammatory state associated with HIV; this is an area of active study [9–11].
The Natural History of HIV Infection
Untreated HIV infection results in persisting and relatively constant levels of viremia and a progressive immune attrition reflected most obviously, but only in part, by a decline in the numbers of circulating CD4 T lymphocytes [12, 13]. The rate of CD4 cell loss varies widely among infected individuals but averages 60–80 cells/mm3 annually [14, 15]. Constitutional symptoms and serious infections and malignancies arise with immune attrition, particularly when the peripheral CD4 count falls below 200 cells/mm3. Many with initial, or acute, primary HIV infection have a 1- to 2-week clinical illness [16, 17]. Following recovery from any symptoms of this acute phase of HIV infection, most are asymptomatic until much later in the disease course [18]. With progressing disease, some experience constitutional signs and symptoms—chronic or recurring fevers, malaise, weight loss, or other evidence of chronic inflammation. Advanced HIV disease, also termed AIDS, when the CD4 count is below 200/mm3, is punctuated by opportunistic infections and malignancies that range from treatable inconveniences to rapidly fatal and irreversible acute illnesses. While the risk of AIDS-related conditions increases sharply with declining CD4 counts, some illnesses associated with HIV and AIDS may occur at high CD4 levels (e.g. tuberculosis, non-Hodgkin lymphoma). Additionally, a number of so-called “non-AIDS” complications, such as “non-AIDS” malignancies, and cardiovascular, liver, kidney, and neurocognitive disease, may occur at relatively high CD4 counts [11, 19–25]. A number of these complications appear to be associated with persistent immune activation and inflammation present in untreated persons with HIV infection, even at high CD4 counts, and are incompletely reversed by ART [26, 27]. Some researchers have suggested that HIV infection results in what may be considered an acceleration of the normal aging process [26, 27]. While some individuals progress from initial infection to death in as little as 12 months, most are relatively stable for a number of years. A very small number of HIV-infected individuals maintain control of HIV without medications and may survive many years with no apparent ill health [28].
HIV disease is staged by the CD4 count, with numbers above 500/mm3 considered in the normal range, while those below 200/mm3 [3] indicate advanced disease or AIDS. As the risk of specific opportunistic diseases correlates closely with the CD4 count, this test is of particular value in patient management. By contrast, levels of HIV viremia are less predictive of disease stage, but may correlate with the rate of disease progression, and may indicate treatment failure.
The HIV Life Cycle
Early targets in the HIV life cycle
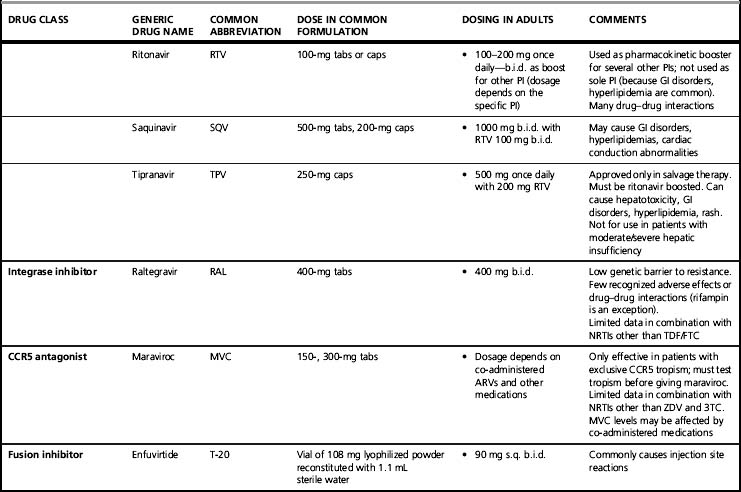
After the viral surface glycoprotein gp120 and the cell surface protein CD4 interact in attachment [29], the CD4 changes its conformation to allow the engagement into this complex of a second cell surface protein, the co-receptor, whose natural function is to act as a chemokine receptor, either CCR5 or CXCR4 [30, 31]. The CD4–gp120–chemokine receptor complex in turn activates the viral gp41, which uncoils, inserts a fusion protein into the cell surface membrane, recoils while tethered to the cell, and approximates the viral and cell membranes, resulting in their fusion [32]. The CCR5 chemokine co-receptor is the target of one ARV class, the CCR5 antagonist. It is the first class directed at a human target. The currently available agent is maraviroc; others are in development. The tethered uncoiled gp41 is the target of the fusion inhibitor enfuvirtide. These “early” acting drugs can actually prevent cellular HIV infection, in contrast to other available ARV drugs.
Middle targets in the HIV life cycle
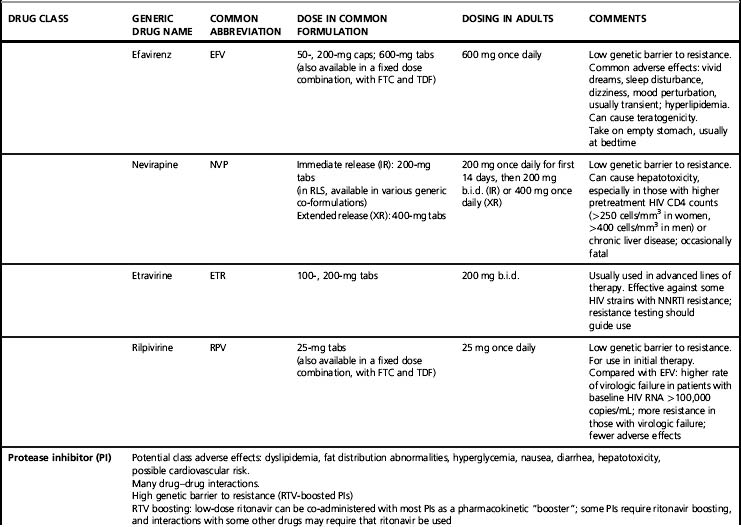
Following membrane fusion, the viral core enters and uncoats in the target cell cytoplasm where the viral genes encoded on the single-strand HIV RNA genome are reverse transcribed into a dual-strand DNA copy [33]. The enzyme that facilitates this, reverse transcriptase, is the target of many ARV drugs, some structural analogs of normal nucleosides or nucleotides [34, 35]. Other drugs that block this enzyme, the non-nucleosides, bind to the enzyme’s active site, but have a chemical structure that does not resemble nucleosides [36, 37]. Reverse transcriptase inhibitors of both types only act following cellular infection by HIV. By convention, the nucleosides (or nucleotides)—like reverse transcriptase drugs—are called the NRTIs while the non-nucleoside agents are called the NNRTIs.
After reverse transcription, the dual-stranded HIV DNA virus is incorporated into the host cell’s DNA via a process mediated by the viral enzyme integrase. Integrase inhibitors target this enzyme [38]. Raltegravir is the first available integrase inhibitor; others are in development.
Late targets in the HIV life cycle
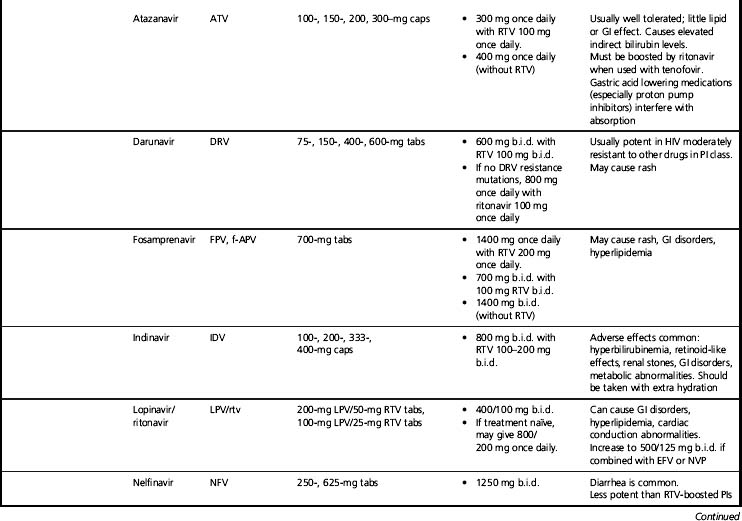
As the new HIV virion forms inside the cell and then buds into the extracellular environment, trimming of the structural or gag-related proteins by HIV protease is necessary for full infectivity. HIV protease inhibitors (PIs) target that enzyme. Most PIs typically are used in combination with low-dose ritonavir. Ritonavir is itself a PI, but in current practice is used only as a pharmacokinetic enhancer of the co-administered PI, to increase plasma levels of that PI and allow added potency and convenience [39]. The co-administration of low-dose ritonavir is commonly called “boosting.” A ritonavir-boosted PI is counted as a single drug as the ritonavir dosage is sub-therapeutic. Potential drug targets in the late life cycle events include gag protein maturation [40] and vif [41], a viral gene that appears to act by inhibiting innate cellular antiretroviral factors.
Components of ARV Regimens
Achieving treatment goals through suppressing HIV replication to the lowest possible levels requires the simultaneous use of multiple ARV drugs. Most initial ARV regimens consist of a dual NRTI “backbone” and a third or “cornerstone” drug [2, 3]. Typically, the “cornerstone” drug is an NNRTI, a PI (usually boosted by low-dose ritonavir), or an integrase inhibitor. Alternative approaches, e.g. regimens composed of three NRTIs, a PI and an NNRTI without NRTIs, or boosted-PI monotherapy, have been attempted but generally have been less potent [42–44]. These are not currently a preferred option for most patients, though additional studies are underway. Each drug in a typical triple-drug combination must be considered on its own in terms of potency, convenience, and toxicity, but the entire regimen must be similarly considered. Designing an optimum regimen must be individualized for each patient. These factors are discussed in greater detail later in this chapter.
Summarizing each ARV drug and all possible regimens of choice is beyond the scope of this review, although Tables 11.1 and 11.2 offer a brief overview of commonly used agents and regimens. Excellent summaries of current ART information are included in guidelines published by national and international organizations. In the United States, both the DHHS [3] and the IAS-USA [2] guidelines are frequently updated. The DHHS guidelines are an especially extensive information resource for many aspects of drug potency, toxicity, and interactions. The IAS-USA also publishes updated guidelines of ARV resistance testing [45], which are extremely useful for planning treatment. Other chapters in this book address HIV biology and important ARV treatment issues including drug toxicity, drug resistance, adherence, and drug interactions. These chapters should be consulted for this crucial information.
Table 11.2 Common antiretroviral regimens using FDC backbones for initial therapy
| Regimen | Advantages | Disadvantages of the regimena |
|---|---|---|
| NRTI backbone: TDF+FTC | NRTI considerations | |
| [TDF+FTC]+EFV | All once daily in one combination pill. Extensive data demonstrating efficacy. Per US guidelines, a recommended regimen for initial therapy | Two drugs with low resistance barrier. Avoid EFV in first trimester of pregnancy or in women who may become pregnant |
| [TDF+FTC]+NVP | Low pill burden (2 pills). NVP effective in preventing HIV transmission in pregnant women. Can be once daily (with NVP XR formulation) | Two drugs with low resistance barrier |
| [TDF+FTC]+RPV | All once daily in one combination pill Low incidence of adverse effects | Two drugs with low resistance barrier. Compared with [TDF+FTC]+EFV, higher rate of virologic failure in those with baseline HIV RNA >100,000 copies/mL; more resistance in those with virologic failure. Little information available on interactions with other drugs |
| [TDF+FTC]+ATV/rtv | All once daily, low pill burden (3 pills). Per US guidelines, a recommended regimen for initial therapy | |
| [TDF+FTC]+DRV/rtv | Can be once daily if no DRV-associated resistance mutations. Per US guidelines, a recommended regimen for initial therapy | |
| [TDF+FTC]+LPV/rtv | Can be once daily | More pills and greater risk of adverse effects than in recommended combinations |
| [TDF+FTC]+SQV/rtv | No once daily option. More pills and greater risk of adverse effects than in recommended combinations | |
| [TDF+FTC]+FPV/rtv | Can be once daily in initial therapy | More pills and greater risk of adverse effects than in recommended combinations |
| [TDF+FTC]+IDV/rtv | No once daily option. Likely has greater toxicity than other regimens | |
| [TDF+FTC]+NFV | No once daily option. More pills and greater risk of adverse effects than in recommended combinations | |
| [TDF+FTC]+RAL | Well tolerated. Few drug–drug interactions. Per US guidelines, a recommended regimen for initial therapy | Two drugs with low resistance barrier; RAL is b.i.d. |
| [TDF+FTC]+MVC | MVC effective only against CCR5 tropic virus; tropism testing must be done before starting MVC. Limited experience with this combination. MVC is b.i.d. | |
| NRTI backbone: ABC+3TC | ABC+3TC (compared to TDF+FTC) may have higher rates of early virologic failure in patients with baseline viral load >100,000 copies/mL | |
| [ABC+3TC]+EFV | All once daily, low pill burden (2 pills). Substantial data show efficacy | Two drugs with low resistance barrier. Both ABC and EFV can cause rash. Avoid EFV in first trimester of pregnancy or in women who may become pregnant |
| [ABC+3TC]+NVP | Low pill burden (3 pills). Can be once daily (with NVP XR formulation) | Two drugs with low resistance barrier. Both ABC and NVP can cause rash |
| [ABC+3TC]+ATV/rtv | All once daily, low pill burden (3 pills) | |
| [ABC+3TC]+ATV | All once daily; avoids RTV. Low pill burden (3 pills) | Lower genetic barrier to resistance than ATV/rtv |
| [ABC+3TC]+LPV/rtv | Can be once daily if no LPV/rtv resistance | See Table 11.1 for LPV/rtv considerations. More pills; greater risk of adverse effects than in recommended combinations, especially with once daily dosing |
| [ABC+3TC]+SQV/rtv | No once daily option. More pills and greater risk of adverse effects than in recommended combinations | |
| [ABC+3TC]+FPV/rtv | Can be once daily in initial therapy | Both ABC and FPV can cause rash. More pills and greater risk of adverse effects than in recommended combinations |
| [ABC+3TC]+DRV/rtv | Can be once daily if no DRV resistance | Both ABC and DRV can cause rash |
| [ABC+3TC]+IDV/rtv | No once daily option. Likely has greater toxicity than other regimens | |
| [ABC+3TC]+NFV | No once daily option. More pills and greater risk of adverse effects than in recommended combinations | |
| [ABC+3TC]+RAL | Few drug–drug interactions. Low pill burden (3 pills) | Limited clinical experience with this combination. RAL is b.i.d. Two drugs with low resistance barrier |
| [ABC+3TC]+MVC | MVC effective only against CCR5 tropic virus; tropism testing must be done before starting MVC. MVC is b.i.d. Limited experience with this combination | |
| NRTI backbone: ZDV+3TC | ZDV may have greater toxicity than TDF or ABC, may have lower CD4 increases than TDF/FTC or ABC/3TC. ZDV is b.i.d. AZT/3TC is the preferred NRTI backbone in pregnant women | |
| [ZDV+3TC]+EFV | Two drugs with low resistance barrier. ZDV+3TC is b.i.d., EFV is once daily. Avoid EFV in first trimester of pregnancy or in women who may become pregnant | |
| [ZDV+3TC]+NVP | All b.i.d. A preferred regimen for preventing mother-to-child transmission in pregnant women | Two drugs with low resistance barrier. No once daily option |
| [ZDV+3TC]+ATV/rtv | ZDV+3TC is b.i.d. | |
| [ZDV+3TC]+DRV/rtv | ZDV+3TC is b.i.d. | |
| [ZDV+3TC]+LPV/rtv | A preferred regimen for preventing mother-to-child transmission in pregnant women | No once daily option. More pills and greater risk of adverse effects than in recommended combinations |
| [ZDV+3TC]+SQV/rtv | No once daily option. More pills and greater risk of adverse effects than in recommended combinations | |
| [ZDV+3TC]+FPV/rtv | No once daily option. More pills and greater risk of adverse effects than in recommended combinations | |
| [ZDV+3TC]+IDV/rtv | No once daily option. Likely has greater toxicity than other regimens | |
| [ZDV+3TC]+NFV | Effective in preventing mother-to-child transmission in pregnant women | No once daily option. More pills and greater risk of adverse effects than in recommended combinations |
| [ZDV+3TC]+RAL | All b.i.d. Few drug–drug interactions | |
| [ZDV+3TC]+MVC | All b.i.d. | MVC effective only against CCR5 tropic virus; tropism testing must be done before starting MVC. No once daily option |
| [ZDV+3TC+ABC] | Low pill burden—one pill b.i.d. | Less potent than regimens with NNRTI or PI component |
Many combinations for initial ART can be constructed using available agents. Each has potential advantages and disadvantages, and the selection of ARV regimens should be individualized. Combinations that have a high degree of efficacy, safety, tolerability, and convenience include those listed above, all of which are recommended by current US and European guidelines (note that alternative combinations may be indicated for the individual patient).
a See Table 11.1 for disadvantages of individual ARVs.
An Overview of Common Components of ARV Regimens
Initial ARV regimens typically comprise a dual NRTI “backbone” and a third or “cornerstone” drug [2, 3].
NRTIs
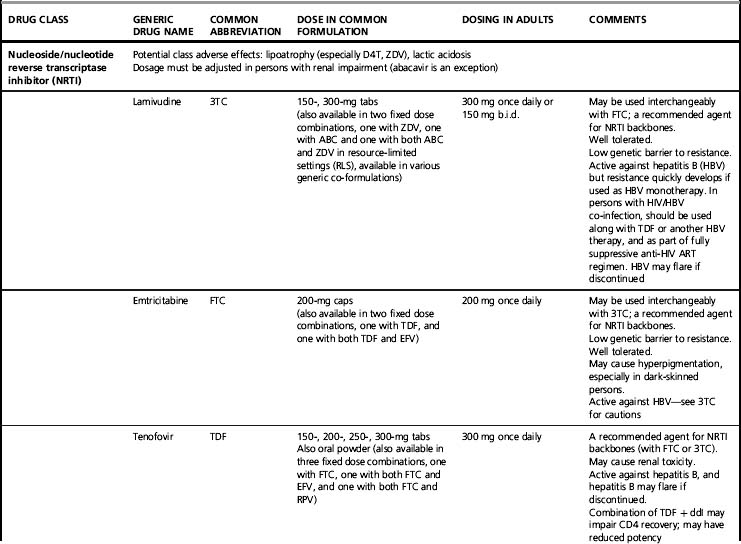
Of all possible two-drug NRTI combinations, several are commonly prescribed, while others are to be avoided and yet others can be useful in specific situations affected by prior toxicity or drug resistance. Preferred combinations are co-formulations of two drugs in a single pill, with either lamivudine or emtricitabine as one of the agents. Co-formulation increases convenience and potentially improves medication adherence [46]. NRTIs as a class may cause lipoatrophy and lactic acidosis, though the risk of these varies widely with specific NRTIs.
Lamivudine (3TC) and emtricitabine (FTC)
Lamivudine and emtricitabine are closely related NRTIs and can be used interchangeably. One or the other typically is included in a dual NRTI backbone. They are very well tolerated, though emtricitabine may cause patchy hyperpigmentation, particularly in dark-skinned individuals. A single mutation, M184V, confers high-level HIV resistance to 3TC and FTC. Both NRTIs are also active against hepatitis B virus (HBV) but in persons with HBV co-infection, should not be used without other HBV-active drugs because HBV resistance commonly develops [47].
Dual NRTI backbones: co-formulations
Tenofovir (TDF) plus emtricitabine (FTC)
This is a potent and convenient one pill, once daily backbone, and is recommended by US [2, 3] and European guidelines [48] as the preferred NRTI combination for most patients. It also is co-formulated with efavirenz as a three-drug combination tablet. Tenofovir usually is well tolerated in short-term use but has been associated with renal toxicity; renal function should be monitored [49]. Questions of potential bone toxicity are under investigation [50, 51]. Tenofovir has interactions, especially with atazanavir (whose levels decrease) and didanosine (whose levels increase). When used with didanosine, increases in CD4 counts may be dampened; this combination should be avoided [52]. Both tenofovir and emtricitabine are active against hepatitis B virus, and in persons with HIV–HBV co-infection this combination is recommended as part of the anti-HIV regimen in to co-treat HBV infection (although emtricitabine is not approved for this indication).
The HIV resistance pattern of tenofovir includes a K65R mutation that can lead to cross-resistance with some other drugs in this class. This mutation is rare if either a thymidine analog, or a potent “cornerstone” drug is co-administered [53]. Thymidine analog mutations (TAMs) may decrease the potency of tenofovir.
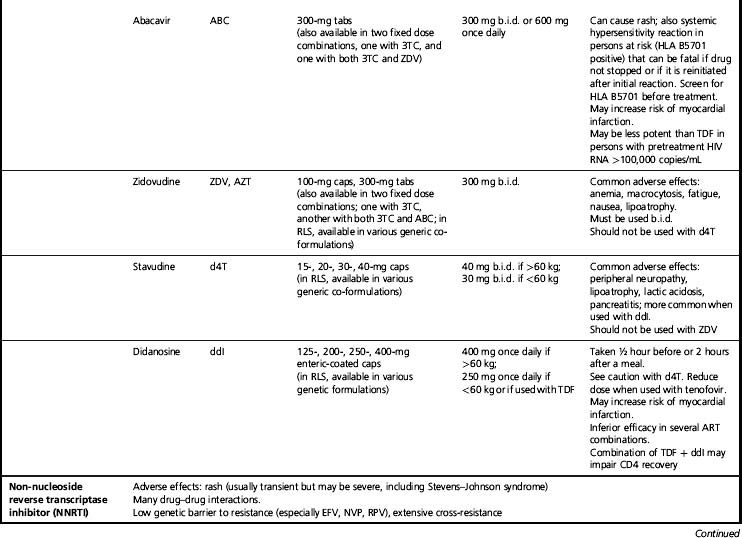
Abacavir (ABC) plus lamivudine (3TC)
This one pill, once daily co-formulation, also has had extensive clinical application [54]. Abacavir is a potent non-thymidine NRTI with no significant drug interactions. It is well tolerated in long-term use but may cause an occasionally severe hypersensitivity reaction in early use characterized by fever, rash, malaise, and, in extreme cases where the drug is continued or reintroduced, circulatory collapse and death [55]. Hypersensitivity to abacavir is closely associated with HLA B*5701, and genetic screening for this allele should be done before treatment with abacavir, and those testing positive should not be given abacavir [3, 56]. Abacavir has been associated with adverse cardiovascular effects in some studies but not others; the possibility of cardiovascular toxicity remains under investigation [57, 58]. In patients with high pre-treatment HIV viral loads (>100,000 copies/mL) one study found that regimens containing abacavir–lamivudine were not as effective in suppressing HIV viremia as those with tenofovir–emtricitabine; this result has not been found consistently [59]. As mentioned above, lamivudine should not be used in persons with HBV infection without other HBV-active drugs because HBV resistance commonly develops. Drug resistance patterns with abacavir are similar to tenofovir with a K65R mutation, but also seen is the L74V mutation. TAMs may decrease the potency of abacavir.
Zidovudine (ZDV) plus lamivudine (3TC)
This combination was the first to be co-formulated and has had extensive use. It must be used twice daily. Zidovudine can cause anemia, sometimes of severe grade. It also may cause nausea, fatigue, facial and peripheral fat loss (lipoatrophy), and other symptomatic adverse effects. Because of toxicity concerns, zidovudine is not recommended in the resource-rich world unless other options are not possible; however, it still is commonly used in resource-constrained areas. For treatment of pregnant women, though, zidovudine remains the preferred NRTI, as it has been extensively studied in the prevention of perinatal HIV transmission [60]. This combination has minimal interactions with other ARV drugs. Zidovudine’s resistance barrier is fairly broad. As mentioned above, lamivudine should not be used in persons with HBV without other HBV-active drugs because HBV resistance commonly develops.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree