Chapter 25 Other HIV-related pneumonias
Frequency
Bacterial pneumonia was a common cause of HIV-related complications in the era before the availability of highly active ART (HAART) and has continued to be a major problem in the developing world. A prospective study of 1,100 HIV-infected patients from 1988 through 1994 in the USA found an incidence of approximately 100 cases per 1,000 person-years (PY), approximately eight times higher than for an age-matched control population [1, 2]. The major pathogens encountered in this study of 521 cases of pneumonia (in which the likely pathogen was identified) were bacterial infection, 44%; P. jiroveci, 42%; tuberculosis, 5%; and other opportunistic infections, 8%. Of the bacterial causes, the most frequent were Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, and Staphylococcus aureus. Atypical agents (Legionella, Mycoplasma pneumoniae, and Chlamydophila pneumoniae) were rarely encountered [3].
Bacterial Pneumonia
Pneumococcal pneumonia
The frequency of pneumococcal bacteremia is estimated to be 150–300 times higher in HIV-infected individuals than in those seronegative for HIV. The increased rate appears to apply to all CD4 cell strata, but is most common in those with low CD4 counts [3, 5]. This frequency has changed substantially in developed countries due to the notable impact of HAART, the induction of herd immunity due to the use of the Prevnar 7 vaccine in children, and the demonstrated impact of PCP prophylaxis in reducing bacterial infections [4, 7].
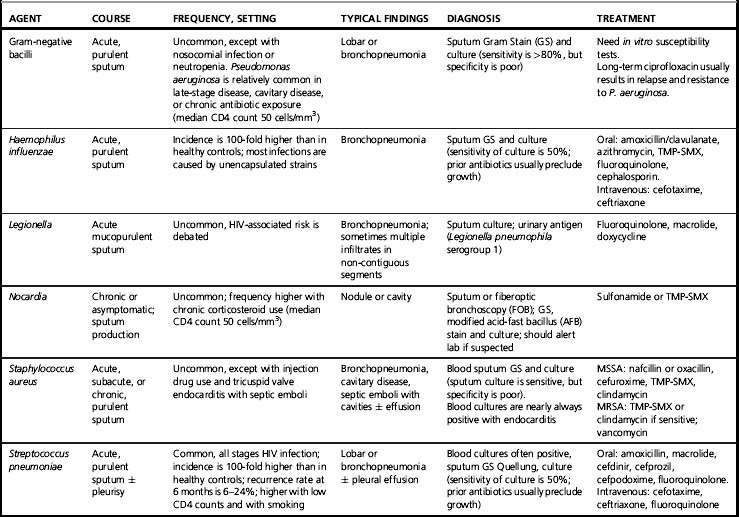
The clinical presentation of pneumococcal pneumonia is similar for patients with or without HIV infection, except for the high rates of bacteremia in HIV-infected patients. Extrapulmonary involvement (meningitis, septic arthritis, and endocarditis) are infrequent. The classic presentation is the acute onset of chills and fever, usually accompanied by cough, dyspnea, and pleurisy. These symptoms clearly distinguish this pulmonary infection from Pneumocystis pneumonia (PCP) and tuberculosis (TB). The chest radiograph demonstrates the usual focal infiltrate (Table 25.2). Cavity formation, atypical infiltrates, and hilar adenopathy are rare and suggest an alternative diagnosis. Most patients produce sputum, which becomes a diagnostic resource using Gram stain, Quellung tests, and culture [9]. As noted, blood cultures are often positive. The urinary antigen assay has a sensitivity of approximately 80% and a specificity of 95% in adults with pneumococcal bacteremia [10]. The treatment of pneumococcal pneumonia is the same for persons with HIV infection as for others.
The preferred drugs are summarized in Table 25.2. In Africa, most strains of S. pneumoniae appear to respond to beta-lactams, which are the preferred drugs. In the USA ceftriaxone or cefotaxime are active against about 94% of strains; if there is a reason to suspect resistance or if the patient is critically ill, most experts recommend a fluoroquinolone, either alone or in combination with one of the preferred beta-lactams. One curious observation is that patients who are critically ill with bacteremic pneumococcal pneumonia involving penicillin-sensitive strains appear to do better with a beta-lactam combined with a macrolide than with a beta-lactam alone [11]. The reason is unclear, but some suspect a role of the anti-inflammatory activity of the macrolide. The duration of therapy is arbitrary; the usual recommended duration is 5–7 days after the patient becomes afebrile. The rate of recurrent pneumococcal bacteremia is high: 8–25% within 6 months. These are generally infections involving new strains rather than relapses, so longer treatment does not appear to be beneficial [12, 13].
Administration of the 23-valent pneumococcal vaccine is recommended in HIV-infected patients, although data demonstrating clinical benefit are sparse. In other populations, the only benefit convincingly shown was a 50% reduction in the frequency of pneumococcal bacteremia, suggesting that patients with AIDS may continue to be a high priority for vaccination, especially when the CD4 count is high enough to allow for an immunologic response [14]. More recent data suggest that the new Prevnar 13 vaccine may be useful in adults at high risk for pneumococcal bacteremia [15].
Haemophilus influenzae
This organism is second to the pneumococcus as a cause of bacterial pneumonia in patients with HIV infection [16, 17]. The frequency of H. influenzae bacteremia is 10- to 100-fold higher in HIV-infected patients. Most cases involve non-typeable strains [17]. This infection is similar to that of pneumococcal pneumonia, with an acute onset of fever, cough, sputum production, and dyspnea. Chest X-rays usually show a bronchopneumonia. The diagnosis is best established by Gram stain and culture of sputum and culture of blood. Approximately 40% of these strains produce beta-lactamase, so amoxicillin is often ineffective. The preferred drugs are a third-generation cephalosporin, a beta-lactam/beta-lactamase inhibitor, fluoroquinolone, azithromycin, or sulfamethoxazole-trimethoprim [18]. The response is generally good, and treatment is continued for 5–7 days after the patient becomes afebrile. Trimethoprim-sulfamethoxazole prophylaxis should prevent this infection, but other forms of PCP prophylaxis will not. Haemophilus influenzae vaccine is not indicated in adults because the rates of this infection are relatively low and the majority involve non-typeable strains not included in the vaccine.
Staphylococcus aureus
Patients with HIV infection have not been clearly defined as being at higher risk for infections involving S. aureus. Those infected with HIV as a result of injection drug use have increased rates of infections involving S. aureus that appear to be independent of HIV. Most of these infections are soft tissue infections at injection sites or pyomyositis [18, 19]. There is a relatively new form of staphylococcal pneumonia that is at present quite rare, but might become more prevalent and is important to recognize. This is the strain that has the Panton-Valentine leukocidin (PVL) recognized in the late 1990s that is often resistant to all beta-lactams, but sensitive to most other drugs active against S. aureus, including trimethoprim-sulfamethoxazole, clindamycin, and doxycycline. It is most frequently associated with severe soft tissue infections, including necrotizing fasciitis and furunculosis. HIV-infected patients infected through homosexual sex or injection drug use are at higher risk for these soft tissue infections. This organism may also cause pneumonia characterized by a fulminant course, with shock, necrosis of the lung, and empyema. As noted, the unique strain of S. aureus involved in these cases in the USA is usually referred to USA300, although similar strains with similar properties are found in other parts of the world. The pathogenic mechanism is not clear, but a characteristic feature of these strains is that they possess the genes for the PVL as well as the mecIV mechanism of methicillin resistance [20, 21]. Optimal treatment of pneumonia is not clear, but many authorities recommend the use of vancomycin or linezolid, often in combination with other drugs such as rifampin or clindamycin [21].
Pseudomonas aeruginosa
Pneumonia with this organism is infrequent, but it is a serious complication of late-stage disease indicating profound immunosuppression [22–24]. Most patients have a CD4 count <50 cells/mm3, and some have additional risk factors such as neutropenia or corticosteroid therapy. Many have bacteremia, and the mortality rate is relatively high. The organism is usually easy to recover from sputum and refractory to eradication despite aggressive antimicrobial therapy. The usual treatment is a combination of antipseudomonal beta-lactams combined with tobramycin. Treatment with an oral fluoroquinolone such as levofloxacin or ciprofloxacin often results in relapse with a fluoroquinolone-resistant strain.
Atypical agents
Chlamydophila (formerly Chlamydia) pneumoniae and Mycoplasma pneumoniae appear to be relatively uncommon in patients with HIV infection [1–3, 16]. A major problem is the limited accuracy of the diagnostic test for these two atypical agents [9]. One study reported results with the standard microimmunofluorescence (MIF) serology; nevertheless, C. pneumoniae accounted for only 13 of 319 (2.5%) of pneumonias in patients with HIV infection [25]. Chlamydophila pneumoniae and M. pneumoniae have not been generally associated with infections in compromised hosts, which presumably accounts for the paucity of cases. By contrast, Legionella has a clear association with compromised cell-mediated immunity, so one would expect more cases with HIV infection [26–29]. One early report indicated a 50-fold increase in the frequency of Legionnaires’ disease with HIV infection, but this has remained an isolated report not substantiated by others. Blatt and co-workers [26] reviewed eight cases of Legionnaires’ disease encountered in the HIV Natural History Study of the US Air Force; the median CD4 count was 83 cells/mm3; five cases were nosocomial; six had coexisting pulmonary pathogens; none acquired this infection while receiving prophylaxis with trimethoprim-sulfamethoxazole; and all responded well to standard therapy with a macrolide. The conclusion is that atypical agents play a minimal role in the etiology of bacterial pneumonia in patients with HIV infection or AIDS.
Diagnostic approach
Key clues to the probability of a bacterial pneumonia in a patient with HIV infection are the features that characterize bacterial pneumonia in other populations: the acute onset and rapid progression, the usual clinical features of cough, sputum, and dyspnea, and the radiographic findings, which almost invariably include a pulmonary infiltrate. Key factors in the assessment are the CD4 count (to evaluate susceptibility to opportunistic pathogens), measures of oxygenation and vital signs (to assess severity of illness), and the characteristic features of the infiltrate [23]. Radiographic features that suggest specific etiologic causes are summarized in Table 25.1. In many cases, this will be the initial presentation, and the CD4 count may be pending when diagnostic and therapeutic decisions need to be made. The absolute lymphocyte count (ALC) may be helpful: an ALC of <1200 cells/mm3 correlates roughly with a CD4 count of <200 cells/mm3. Patients with advanced HIV infection may also demonstrate evidence of chronic disease with anemia, hypoalbuminemia, weight loss, etc. If HIV infection is suspected but has not been diagnosed, a rapid serologic test can be helpful. This test and the CBC are generally available in virtually all parts of the world.
Table 25.1 Correlation of chest X-ray changes and causes
| Change | Common | Uncommon |
|---|---|---|
| Consolidation | Pyogenic bacteria, Kaposi’s sarcoma, cryptococcosis | Nocardia, M. tuberculosis, M. kansasii, Legionella, Bordetella bronchiseptica |
| Reticulonodular infiltrates | Pneumocystis jiroveci, M. tuberculosis, histoplasmosis, coccidioidomycosis | Kaposi’s sarcoma, toxoplasmosis, CMV, leishmaniasis, lymphoid interstitial pneumonitis |
| Nodule | M. tuberculosis, cryptococcosis | Kaposi’s sarcoma, Nocardia |
| Cavity | M. tuberculosis, S. aureus (IDU), Nocardia, P. aeruginosa, cryptococcosis, coccidioidomycosis, histoplasmosis, aspergillosis, anaerobes | M. kansasii, MAC, Legionella, Pneumocystis jiroveci, lymphoma, Klebsiella, Rhodococcus equi |
| Hilar nodes | M. tuberculosis, histoplasmosis, coccidioidomycosis, lymphoma, Kaposi’s sarcoma | M. kansasii, MAC |
| Pleural effusion | Pyogenic bacteria, Kaposi’s sarcoma, M. tuberculosis (congestive heart failure, hypoalbuminemia) | Cryptococcosis, MAC, histoplasmosis, coccidioidomycosis, aspergillosis, anaerobes, Nocardia, lymphoma, toxoplasmosis, primary effusion lymphoma |
To establish an etiologic diagnosis, standard tests for patients sufficiently sick to require hospitalization are blood cultures and, in many centers, Gram stain and culture of expectorated sputum [9]. Although emphasis has been placed on the probability of bacterial pneumonia in patients with acute onset of symptoms and other characteristic features, there must be a continued concern for the possibility of PCP in any patient who has a low CD4 count, substantial reduction in oxygenation, lack of PCP prophylaxis, and/or characteristic features on chest radiograph. In these cases, it is important that the diagnostic evaluation include bronchoscopy or induced sputum examination, or, if the probability is sufficiently great, empiric treatment that may or may not include agents active against common bacterial pathogens. The same vigilance applies to TB, particularly in countries where this is highly endemic. The expectorated sputum needs to be evaluated by stain and culture for AFB. Since AFB smears have a sensitivity of only about 50% in HIV-infected patients, it may be important to consider empiric treatment here as well. This problem will diminish with availability of polymerase chain reaction (PCR) assays to rapidly detect M. tuberculosis and multi-drug resistant tuberculosis (MDR-TB) [25].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree