Osmolality and Passive Transport
QUICK LOOK AT THE CHAPTER AHEAD
Passive transport depends on the concentration of fluids. Two examples of passive transportation are diffusion and osmosis. Both types of passive transportation and a comparison of osmolality and osmolarity are explained in this chapter.
Frequently, the terms osmolarity, the osmolar concentration of 1 L of solution, and osmolality, the osmolar concentration in 1 kg of water, are used interchangeably. This switching of terms is accepted because 1 L of water is equal to 1 kg in weight. However, osmolarity is used most often when referring to solutions and fluids outside the body and osmolality when referring to fluids inside the body.
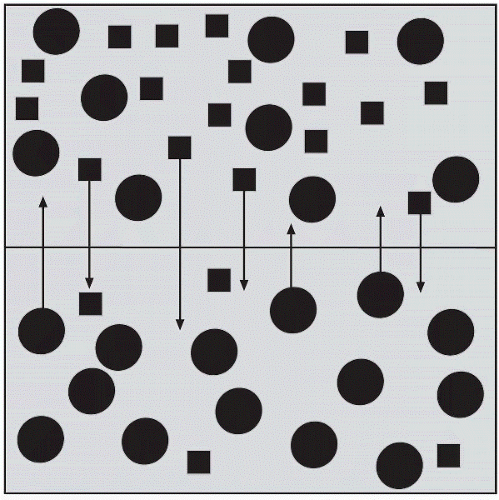
The semipermeable membrane of the cell allows a constant movement of some solutes, whereas others remain confined within their boundaries. Whether confined or free to move, a solution’s osmotic concentration is determined by the amount of dissolved particles. The normal
range for serum osmolality is 275-295 mOsm/kg (milliosmols per kilogram). Blood values that fall below this range indicate a hypoosmolar state and those that are greater than 295 mOsm/kg are considered a hyperosmolar state. The following formula is used to determine serum osmolality:
range for serum osmolality is 275-295 mOsm/kg (milliosmols per kilogram). Blood values that fall below this range indicate a hypoosmolar state and those that are greater than 295 mOsm/kg are considered a hyperosmolar state. The following formula is used to determine serum osmolality:
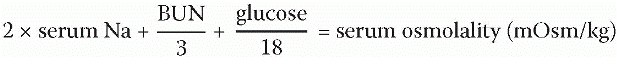
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree