Chapter 10 Options for women around fertility and reproduction
This chapter offers a brief introduction to fertility and reproductive issues faced by women who may not achieve pregnancy without assistance. It will introduce you to some of the physical methods of assisted reproduction, and some of the ethical dilemmas associated with fertility and reproduction technology.
INTRODUCTION
Demographic, economic, social and administrative changes have all had a role in fertility transition (Caldwell 1999). A sustained fall in fertility is a relatively recent phenomenon. In the late 18th century a sustained fall in fertility began in France, and fertility declines became general in western and central Europe, as well as in English-speaking settlement countries, in the last quarter of the 19th century (Caldwell 1999). The reasons were complex (Caldwell 1999). Fertility decline has never been an unconscious social process; advocacy and organisation have been important. From 1950 to 1965, the Western world population increased by 34% and that of developing countries by 39% (Caldwell 1999). In developing countries, in the 15 years from the early 1950s until the late 1960s, life expectancy at birth had risen from under 41 years to over 52 years and the annual population growth rate had climbed from 2.0% to 2.5%, while the total fertility rate (the average number of lifetime births per woman at the age-specific birth rates at a specified date) had remained at just over six. Social theorists began to proclaim that it was unique characteristics of the Western family that had allowed or ordained fertility control, and others suggested that the socioeconomic gap between the late 19th century West and the mid-20th century Third World was greater than had been thought. But by 1970 it was clear that there had been a widespread fall in fertility from about 1965 in both Latin America and Asia (Caldwell 1999).
WORLD POPULATION TRENDS
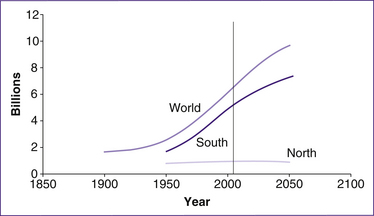
Since 1968, when the United Nations’ Population Division predicted that the world population, now 6.3 billion, would grow to at least 12 billion by 2050, the agency has regularly revised its estimates downwards. It now expects world population to plateau at 9 billion in 2050. We are experiencing a profound demographic shift as a result of two forces: increasing longevity and declining fertility. The best-known example of shrinkage is in Italy, where women were once symbols of fecundity. By 2000, Italy’s fertility rate was western Europe’s lowest, at 1.2 births per woman. Its population is expected to drop by 20% by mid-century. Denmark was below population replacement level in 1970 at 2.0 births per woman, and slid to 1.7 by 2001 (Kurjak & Carrera 2005). (The level required by a population to replace itself in the long term, without migration, is 2.1 births per woman.)
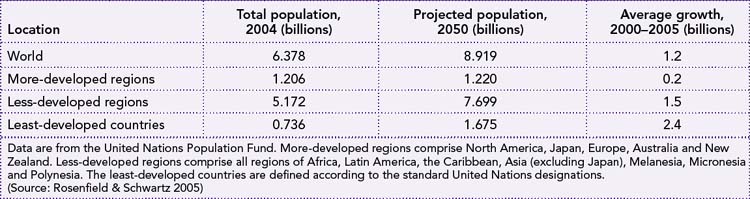
Figure 10.1 shows the projected rise in world population to 9.2 billion in 2050, representing an increase of 2.7 billion over the 2005 population of 6.3 billion. Nearly all of this future growth is predicted to occur in the ‘South’— that is Africa, Asia (excluding Japan, Australia and New Zealand) and Latin America—where population size is projected to increase from 5.3 billion to 7.9 billion between 2005 and 2050. In contrast, in the ‘North’ (Europe, Northern America, Japan and Australia/New Zealand), population size is forecast to
remain virtually stable, growing slightly from 1.22 to 1.25 billion between 2005 and 2050. This occurs because population growth follows what is known as demographic transition (Bongaarts 2009).
Demographic transition usually reflects changes in societies as they move from being dependent on agriculture to becoming industrial societies. At the beginning of the demographic transition, population growth (which equals the difference between the birth and death rate in the absence of migration) is near zero as high death rates more or less offset the high birth rates typical of agrarian societies before an industrial revolution. Then, as the society develops through industrialisation, the population growth moves towards zero as birth and death rates reach low levels and again begin to balance out. Figure 10.1 reflects the differences between the already industrialised nations (North on the graph) and the slower industrialisation occurring in countries such as Africa, Asia and Latin America (South on the graph) (Bongaarts 2009).
However, the assumption that demographic transition from high to low birth rates occurs only as a result of exogenous social and economic forces is contested through our clearer understanding of the many barriers that separate women from the knowledge and technologies they need to manage their childbearing within a human rights framework. When viewed through a human rights framework, the opportunity to slow population growth draws on a number of scientific disciplines such as ecology, climate change and peace and conflict studies—disciplines that have not traditionally played a large part in the debates on population and family planning (Potts 2009). Contraception and safe abortion together have lowered family size even in poor, illiterate communities. Making family planning available in resource-poor settings should be an achievable and feasible global intervention (Prata 2009).
Demographics of New Zealand women
Although the age distribution of mothers may have changed over time (Fig 10.2), the percentage of births among each ethnic group has changed very little.
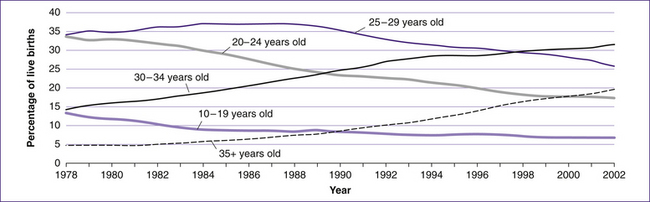
Figure 10.2 Age of mothers at the time of giving birth in New Zealand, 1978–2002
(based on NZHIS 2004)
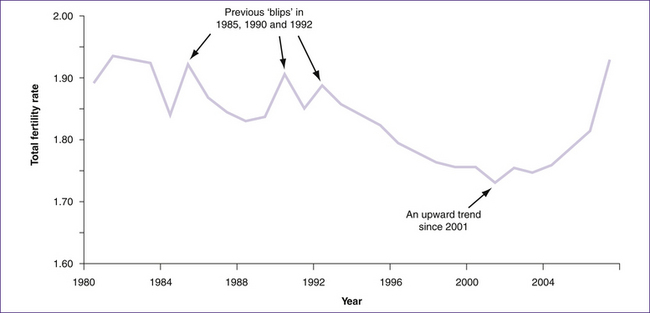
The annual fertility rates for 2009 indicate that New Zealand women average 2.18 births per woman, up from 2.15 in 2008. However, fertility rates of close to or higher than 2.1 births per woman need to be sustained over many years before ‘replacement level’ fertility—the number of babies a woman would need to have during her lifetime to replace both herself and her partner—can be claimed. Since 1980, fertility in New Zealand has been slightly below replacement level, with the exception of short periods around 1990 and the present (Statistics New Zealand 2009).
Demographics of Australian women
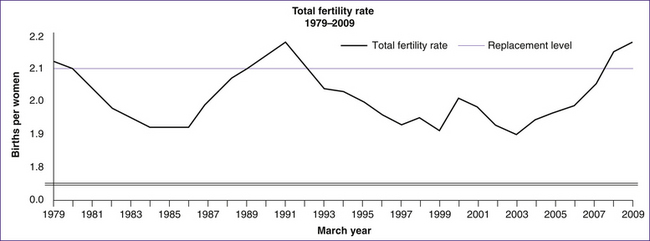
In Australia the estimated total fertility rate of 1.93 babies per woman is the highest since the early 1980s. This is not a one-off event, as fertility rates have been generally rising for the last six years (Fig 10.5). Overall, the evidence suggests that after its long downward trend after the Second World War, Australia’s fertility rate may have stabilised at around 1.75 to 1.9 babies per woman. The current trend in fertility rates appears to be similar to that observed in other low-fertility countries that have experienced slight recoveries in their fertility rates in recent years, including England and Wales (up from 1.6 in 2001 to 1.9 in 2007), Norway (up from 1.8 in 2002 to 2.0 in 2008), Scotland (up from 1.5 in 2002 to 1.7 in 2007) and Sweden (up from 1.5 in 1999 to 1.9 in 2008) (Lattimore & Pobke 2008).
Delayed pregnancy
The choice of delaying pregnancy has become the norm for many women in developed countries. Among some women, however, achieving pregnancy may be difficult or impossible at a later time. Australian and New Zealand women, like women in most developed countries, are
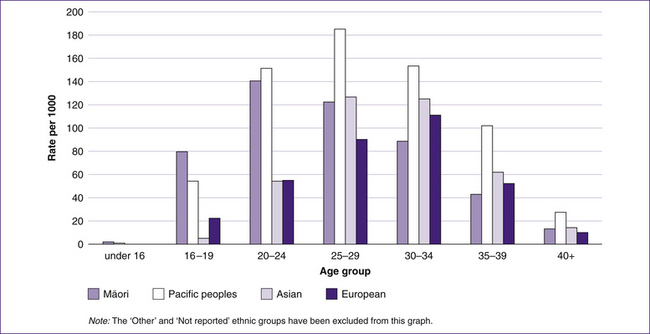
Figure 10.3 Age group and ethnicity of mothers in New Zealand giving birth in hospital in 2002
(based on NZHIS 2004)
delaying childbirth until their thirties and forties. In 1971 the average age of Australian and New Zealand women at the time of giving birth was 25.4 years, but it has consistently increased since then, reaching a peak of 30.7 years in 2007 in Australia and 30 years in New Zealand in 2009 (ABS 2008; Laws & Sullivan 2004; NZHIS 2004; Statistics New Zealand 2009).
Generally, since 1971 the trend in birth rates among women in their thirties has increased while decreasing for the younger age groups in industrialised societies such as Australia and New Zealand. Several causative factors have been identified, such as delayed partnering, higher education, increased employment opportunities for women, longer life-expectancy and improved access to and availability of contraception. These factors promote later childbearing by lowering the costs associated with exiting and re-entering the workforce, reducing the financial risks involved in family formation, and enabling parents to negotiate a better balance between work and caring responsibilities (Lattimore & Pobke 2008). At the same time, advances in assisted reproductive technologies have improved pregnancy rates among infertile couples, adding to the growing group of primiparous women aged 35 years and older.
Women in Australia and New Zealand, along with women elsewhere in the developed world, have had the opportunity to delay childbearing since effective contraception became available in the 1960s. However, because fertility decreases with increasing maternal age, a slow but steady decrease in fertility is observed in women aged between 30 and 35 years, followed by an accelerated decline among women aged over 35 years. This combination of delayed childbearing and reduced fecundity with increasing age has resulted in an increased number and proportion of women greater than or equal to 35 years of age seeking assisted reproductive technology (ART) treatment (Alviggi et al 2009). Over the last five years, the number of ART procedures has increased on average by over 10% per year in Australia and New Zealand. Latest estimates indicate that 3.1% and 1.8% of babies born in Australia and New Zealand respectively are as a result of ART treatment (Wang et al 2009).
The risks associated with ART may include poorer neonatal outcomes such as preterm birth, low birthweight, stillbirth and neonatal deaths (Helmerhorst et al 2004; Wang et al 2009). Infertility treatment is also an independent risk factor for caesarean section among primiparous women aged 40 and above (Sheiner et al 2001).
FERTILITY
The total fertility rate refers to the number of babies a woman could expect to bear, on average, during her lifetime if she experienced current age-specific fertility rates throughout her childbearing life (see Box 10.1).
Box 10.1 What is total fertility rate (TFR)?
• In any given year, fertility rates can be calculated for women of different ages. These ‘age-specific’ fertility rates are calculated for women from 15 to 49 years (with any births to women outside these ages being included in the fertility rates of 15-year-olds and 49-year-olds respectively).
• The TFR is a simple (non-weighted) sum of these rates divided by 1000.
• The TFR is equivalent to the average number of children that would be born to a woman over her lifetime were she to experience current age-specific fertility rates through her lifetime.
(Source: Lattimore & Pobke 2008)
In 2008, the highest fertility occurred in women aged 30–34 years, at a rate of 126.6 babies per 1000 women in Australia and 125 births per 1000 women in New Zealand, continuing the trend of the previous five years. The main decline in the fertility rate over the past 20 years has occurred among the 20–24 and 25–29 age groups. Meanwhile, fertility has continued to increase in women aged 40 and over (ABS 2008), and ART has played a role in this.
In Australia, teenage fertility was 16.0 babies per 1000 women in 2007, slightly higher than in 2006 (15.3 babies per 1000 women). In New Zealand, in 2008 the fertility rate for women aged 15–19 years was 32 births per 1000, half the 1969 rate (65 per 1000). Among women aged 15–19 years who registered a baby in the March 2009 year, around two-thirds were aged 18 or 19 years (Statistics New Zealand 2009).
‘Fertility’ in the colloquial sense of the word denotes ‘reproductive capacity’ (rather than the demographic sense of ‘number of live births’). Aside from some medically determined causes of sterility, the reproductive capacity of a couple cannot be measured directly. Rather, it has to be assessed stochastically: by whether pregnancy occurs and how long it takes the couple to conceive (Sallmen et al 2005, p 494).
The ability to preserve fertility with various methods has become a key issue for some women. Although the need is most pressing among women with cancer, the same therapeutic options may be available for many other women who are reaching an advanced reproductive age. However, in this group, the use of the available techniques is controversial and should be considered experimental (Lobo 2005, p 64).
Physiological factors affecting fertility
The peak number of oocytes in the female infant occurs at about 20 weeks gestation, when there are somewhere between 6 million and 7 million oocytes. Then, by the time of birth, the number has reduced to 1 million to 2 million oocytes; by puberty 400,000 oocytes remain and by the time a woman is 37 years old, she reportedly has approximately 25,000. This number drops over the years until at menopause (around 50 years of age for most women) a woman will have about 1000 oocytes left in her body (Lobo 2005, p 65). As a woman grows older, the depleted oocytes undergo atresia through apoptosis or necrosis (Nwandison & Bewley 2006).
The mechanism underlying this process is poorly understood and involves multiple factors encoded by genes on the X chromosome, as well as on autosomes (Simpson 2000). Although environmental factors may be important, genetic factors predict 44% to 87% of the variance in the age at menopause (de Bruin et al 2001; van Asselt et al 2004). In healthy women, at approximately 37.5 years of age an accelerated atresia of the oocytes begins (Gougeon et al 1994). This accelerated loss is poorly understood and is often associated with a small monotropic rise in the level of follicle-stimulating hormone (FSH) and decreased fecundity (Alviggi et al 2009; Scott et al 1989; Wood et al 1992), as well as an increased risk of aneuploidy. The subtle increase in the level of FSH is thought to increase atresia, which is coupled with an accelerated loss of follicles and a further increase in FSH, thus resulting in a positive-feedback loop (Erickson 2000).
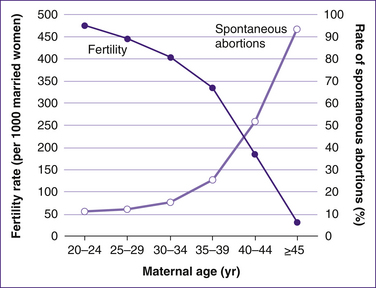
Women who are destined to go through menopause at the age of 45 years (10% of the population) might be expected to have accelerated atresia and reduced fecundity beginning at the age of 32 years; however, this hypothesis has not been proved. Although it is clear that women with a strong family history of early menopause would be at risk for reduced fecundity at an earlier age, no data suggest at what age fecundity decreases. As atresia continues, both the number and the quality of oocytes fall below a critical level. It is well recognised that there is a decrease in the quality of the oocytes with advancing maternal age, as shown by the higher incidence of chromosomal anomalies such as aneuploidies which in turn reduce the chances of successful fertilisation, implantation and early embryo development (Nwandison & Bewley 2006). This increase in the rate of aneuploidy is related at least in part to problems of the meiotic spindle, resulting in nondisjunction, and this process leads to a greater risk of spontaneous abortion once pregnancy occurs.
Various environmental factors and toxic exposures may also affect the age at menopause. The association between environmental factors such as smoking and diet are reported to shorten the functional life span of a woman’s ovaries. Diet may play a role in the occurrence of early menopause (Baird et al 1998). Cigarette smoking is one of the most common and important factors that affects follicle maturation, due to the compounds in tobacco which exert a deleterious effect on follicle maturation (Freour et al 2008; Soares & Melo 2008).
Factors affecting age-related male fertility are summarised in Box 10.2.
Box 10.2 Men, ageing and fertility
• Age-related changes occur in the testes. Leydig cells decrease in number and changes also occur in the basal membranes, seminiferous tubules and tunica albuginea. These result in changes in spermatogenesis, both reduced sperm count and increased genetic abnormalities.
• Those genetic abnormalities associated with abnormal sperm tend to result in early abortion rather than live birth. Chromosomal anomalies that are known to be due to paternal origin, such as the XYY karyotype and 45XO (Turner’s syndrome), have not been shown to be related to paternal age.
• It is becoming clearer that with advancing paternal age (50 years or more), the risk of early or late fetal loss is almost double compared with that of younger fathers after adjusting for maternal age and other maternal variables.
• The risk of early pregnancy loss is compounded if both maternal age is over 35 years and paternal age is over 40 years.
(Source: Nwandison & Bewley 2006)
The body fat connection
That excessive exercise or undernutrition can postpone puberty, reduce fertility or prevent menstruation was first discovered by Rose Frisch in her pioneering studies beginning in the late 1960s (see Box 10.3). Her work describing the reproductive consequences of an altered mass of fat were largely ignored or treated with scepticism. Her hypothesis—that a critical mass of body fat is the crucial trigger of gonadotrophin secretion, both in developing girls and in mature women during reproductive life—was initially based on detailed analysis of worldwide demographic data and was later supported by highly focused clinical investigation (Reichlin 2003).
In otherwise healthy young women, a critical mass of body fat is the essential trigger of cyclical pituitary– ovarian function. A successful pregnancy requires approximately 50,000 calories stored in the form of fat. A critical body-fat mass determines the onset of menses. Excessive leanness, as in people with anorexia nervosa and some competitive athletes and professional dancers, delays the onset of puberty; after menarche has been established, excessive leanness can cause impaired ovulation, infertility and amenorrhoea. Oestrogen deficiency induced by excessive exercise (and decreased fat mass) is associated with premature osteoporosis, even in runners in whom bone formation has been stimulated by exercise. On the positive side, women who have been extremely active athletically have a much reduced risk of breast cancer, presumably because they have been exposed to lower levels of oestrogen over time. These insights have been invaluable for the evaluation of women with delayed puberty and amenorrhoea, and they have important social and medical implications for dancers and athletes. Though less well-documented, critical body-fat mass is probably also important in determining pituitary gonadal activity in men (Reichlin 2003, p 870).
Time to pregnancy refers to the number of menstrual cycles it takes a couple to conceive. Fecundability, in turn, is a couple-specific probability of conceiving a recognised pregnancy per menstrual cycle, given no contraception (Sallmen et al 2005, p 494). The characteristics of ‘infertile’ and ‘infertility’ are made in reference to couples who try for more than a year to conceive. Many infertile couples will eventually conceive, but a sterile subset cannot conceive without medical intervention (Sallmen et al 2005, p 495).
Given the nature of women’s emancipation and their presence in the workforce, there is a relationship between public policies (maternity leave, income tax regulations), workplace conditions (part-time opportunities and the flexibility of work hours) and the availability of affordable non-parental child care that enables mothers to participate in the workforce (Hank & Kreyenfeld 2003).
A growing body of research suggests a changing, now positive, relationship between women’s education or employment and fertility with the advent of social contexts that allow women to combine childrearing and employment. Access to affordable child care is essential for this to occur (Hank & Kreyenfeld 2003).
OLDER WOMEN AND CHILDBIRTH
Most women over age 35 have healthy pregnancies and healthy babies. According to US data, the number of first births per 1000 women aged 35 to 39 years increased by 36% between 1991 and 2001, and the rate among women aged 40 to 44 years leapt by a remarkable 70%. In 2002, 263 births were reported in women between 50 and 54 years of age (Heffner 2004).
Very advanced maternal age, defined as maternal age of 45 years or greater at the time of delivery, has important consequences for both mother and baby. Currently, 0.1% of all Australian and New Zealand women giving birth are in this age category (ABS 2008; Statistics New Zealand 2009). Given the trend towards delayed childbearing and the increasing availability of assisted reproductive techniques, women aged 45 and over may increasingly seek advice about the risks of embarking on a pregnancy (Callaway et al 2005).
The effect of maternal age on the outcome of pregnancy may be best assessed by examining five specific factors (Heffner 2004) that can negatively affect the desired outcome of a pregnancy—a healthy mother and baby. These are:
It is not unusual for a woman in her mid-thirties or older to take longer to conceive than a younger woman. Age-related decline in fertility may be due, in part, to less-frequent ovulation or to problems such as endometriosis, in which tissue similar to that lining the uterus attaches to the ovaries or fallopian tubes and interferes with conception. While women over age 35 may have more difficulty conceiving, they also have a greater chance of bearing twins. The likelihood of naturally conceived (without fertility treatment) twins peaks between ages 35 and 39, then declines.
‘Miscarriage is defined as spontaneous pregnancy loss before the 20th week of gestation. Karyotyping of the products of conception after miscarriage indicates that about two-thirds are chromosomally abnormal’ (Heffner 2004). Miscarriage (spontaneous abortion) is the most common complication in pregnancy, with 15%–20% of clinically recognised pregnancies aborting spontaneously (Andersen et al 2000). There is a strong relationship between maternal age and miscarriage rates. At 20 years of age, the rate is about 10%. It increases to a high of more than 90% among women aged 45 years or older. This high miscarriage rate contributes significantly to decreasing fertility among older women (Heffner 2004, p 1927).
The success rate in using donor eggs from younger women for in vitro fertilisation supports the hypothesis that deterioration occurs in the quality of the ova with advancing maternal age (Heffner 2004).
of either the mother or the fetus is in jeopardy. ‘The risk of hypertensive complications of pregnancy increases steadily as women age; such complications are twice as likely among women aged 40 years or older as among younger women’ (Heffner 2004, p 1928).
How should we counsel young women when they ask about their reproductive choices? Generally speaking, the decade between 25 and 35 years of age would seem to be ideal. A woman’s education is typically complete, she has usually gained some experience in her professional arena, and pregnancy is at its safest. For women between 35 and 45 years of age for whom earlier childbearing was not an option, this decade remains safe enough that maternal age alone should not be a contraindication to childbearing. However, women do face decreasing fertility and a moderate increase in the risks of miscarriage and chromosomal abnormalities as they pass 40 years of age. Perimenopausal and postmenopausal pregnancy remains an option for those women who are lucky enough to find themselves healthy and sufficiently wealthy to pursue it (Heffner 2004, p 1929).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree