Chapter 16 Ocular manifestations of AIDS
Epidemiology
Although several of these conditions constitute AIDS-defining illnesses, most are not reportable diseases and the overall incidence of ocular complications is not precisely known. In general, however, ocular involvement in a person with AIDS is much more common in advanced disease—that is, patients with a CD4 count < 200 cells/mm3. In the pre-ART era, up to 49.1 events of vision loss to 20/200 or worse occurred per 100 eye-years, with the incidence of legal blindness as high as 14.8 per patient-year. The cost of treatment for, and loss of economic productivity from, ocular diseases is substantial [1].
Non-infectious Retinal Vasculopathy
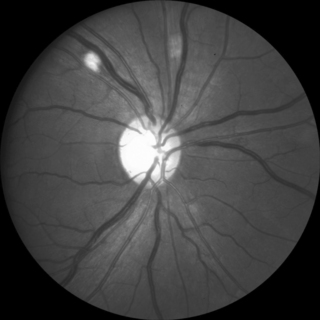
The most common ocular manifestation of HIV infection is a non-infectious retinal microvasculopathy (Fig. 16.1) [2]. The cause is unknown but is probably multifactorial; vascular sludging likely plays an important role. Clinical features include cotton-wool spots and, less commonly, intraretinal hemorrhages, Roth spots, and capillary non-perfusion. Vision is usually unaffected, and patients are otherwise asymptomatic. The cotton-wool spots are evanescent and appear clinically and histologically similar to cotton-wool spots found in other diseases such as diabetes. The significance of the non-infectious retinopathy is the correlation with the degree of immunosuppression. In one study, the retinopathy was found in 45% of patients with a CD4 count < 50 cells/mm3 compared with only 16% of patients with CD4 counts > 50 cells/mm3 [3]. Furthermore, the finding of a cotton-wool spot in a patient on routine examination with no other evident explanation (diabetes, hypertension, etc.) should prompt consideration for HIV testing.
There appears to be a cumulative dysfunction of the inner retina in patients with HIV disease, even in those taking ART, who have not had ocular opportunistic infections [4]. It is not clear whether this phenomenon represents an AIDS-associated primary neuropathy or secondary damage from non-infectious microvasculopathy. The retinopathy may be an indicator of vascular disease elsewhere in the body, in that the presence of non-infectious retinal microangiopathy has been significantly associated with mortality [5].
Retinal macrovasculopathy, including retinal artery and vein occlusions, occurred in 1.3% of nearly 2,500 consecutive HIV-infected patients [6]. There was a strong association between non-infectious retinal microvasculopathy and retinal vein occlusions (odds ratio 5.76). Complications, including severe vision loss, were common, with vision of 20/200 or worse in 40% of eyes. Hypertension or thrombotic disease was common in affected patients.
Diseases of the anterior segment
While there are no AIDS-defining corneal infections, and with the exception of herpes zoster ophthalmicus their incidence does not appear to be significantly greater than in immunocompetent individuals, the presence of certain conditions should prompt consideration of possible immunosuppression. These include multiple molluscum contagiosum lesions, herpes zoster ophthalmicus in young patients [7], multiple or bilateral herpes simplex virus lesions, and microsporidiosis. Jeng et al. have more extensively discussed anterior segment complications of AIDS [8].
Molluscum contagiosum virus (MCV) causes a severe, typically unilateral follicular conjunctivitis in otherwise healthy individuals. In contrast, MCV infection in patients with HIV infection tends to produce much more numerous and larger, often confluent lesions, but without itching or conjunctivitis, even when lesions are found on the ocular surface [9]. The lesions usually fade away with immune recovery, although patients with elevated CD4 counts can develop immune recovery inflammatory syndrome [10]. The differential diagnosis of cutaneous MCV includes bacillary angiomatosis and disseminated histoplasmosis or cryptococcosis. Treatment for cosmetically bothersome lesions includes surgical excision, chemocautery, or cryoablation.
Herpes simplex virus (HSV) can cause periocular, conjunctival, or corneal lesions. The disease is usually unilateral. Cutaneous vesicles can cross dermatomal distributions and the corneal epithelial lesions often have prominent dendrites, two features that help distinguish HSV from zoster keratitis. One large retrospective study that compared the incidence and clinical course of HSV keratitis in HIV-infected and HIV-uninfected patients found no difference in the incidence or type (epithelial or stromal) of keratitis, peripheral versus central location, or time needed for treatment response. However, recurrences were nearly 2.5 times more frequent in the HIV-infected patients [11]. Therapy includes topical trifluridine or ganciclovir or systemic acyclovir or similar drugs. Many ophthalmologists recommend secondary prophylaxis with oral antiviral therapy to reduce the risk of recurrence.
Varicella zoster virus (VZV) can cause cutaneous, conjunctival, or corneal disease in addition to intraocular disease (discussed below). The V1 dermatome is affected in herpes zoster ophthalmicus (HZO) and is the single most commonly affected dermatome in patients with shingles, but multidermatomal distribution is not uncommon. Keratitis, uveitis, and post-herpetic neuralgia are all more common in HZO in HIV-infected patients. These patients should be monitored closely after the development of HZO because of an increased risk of developing acute retinal necrosis [7]. Therapy includes intravenous acyclovir or high-dose oral valacyclovir; topical antivirals are ineffective for zoster keratitis, but topical corticosteroids may be necessary to control uveitis or stromal keratitis.
Microsporidiosis due to Encephalitozoon species can cause a chronic keratoconjunctivitis in HIV-infected patients with low CD4 counts. Symptoms include tearing and foreign body sensation. Clinical features include a bilateral papillary conjunctivitis with prominent hyperemia and diffuse punctate corneal epithelial erosions with intraepithelial infiltrates. In contrast to HIV-uninfected patients with microsporidiosis, the corneal stroma is not affected. The chronicity of the disease may be due to concurrent upper respiratory colonization. The diagnosis requires corneal scraping for Gram stain or electron microscopy. Therapy includes ART to improve host immunity, topical fumagillin, and systemic itraconazole or albendazole to control nasopharyngeal colonization [12].
A variety of miscellaneous anterior segment disorders have been reported. Dry eye is more common in HIV-infected patients, with nearly 18% of patients affected in one study [13]. As dry eye of any cause can increase the risk of contact lens-related problems, HIV-infected patients should be monitored closely for keratopathy and treated with supplemental ocular lubricants as needed. Whether HIV-infected patients are at increased risk for complications of keratorefractive surgery, or if such surgery poses a risk of HIV transmission due to viral particles released in the laser plume, is unclear.
Use of both inhaled crack cocaine and methamphetamine has been associated with infectious and non-infectious corneal ulceration. Putative mechanisms include direct toxic epitheliopathy, neurotrophic keratopathy, alkaline chemical keratopathy, and/or eye rubbing. Atypical pathogens are common [14], even though the conjunctival flora in patients with AIDS appears to be similar to that in HIV-uninfected individuals.
Atopic dermatitis and blepharitis may be more common or more severe in HIV-infected patients than in the general population, although definitive studies are lacking. Treatment for these conditions is similar to that for patients without HIV infection, although topical tacrolimus or other T-cell inhibitors should be avoided if possible because of the possible increased risk of Kaposis’ sarcomas [8].
Pathanapitoon et al. examined 40 HIV-infected patients with uveitis and found 13/40 patients (32%) had detectable HIV-1 RNA [15]. The intraocular HIV load was greater than that found in plasma in three patients undergoing intraocular surgery, all of whom had bilateral anterior uveitis and/or vitritis without retinal lesions, with no identifiable cause of the uveitis otherwise noted. The uveitis in all three patients resolved after initiation of ART.
Disorders of the posterior segment
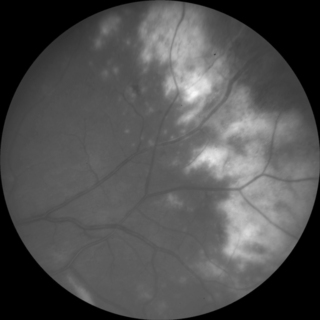
Cytomegalovirus retinitis is the most common ocular opportunistic infection in patients with AIDS. In the pre-ART era, more than 30% of patients with AIDS and advanced immunosuppression developed CMV retinitis, and median survival after the diagnosis was on the order of one year [2]. The infection spreads in a “brushfire” pattern throughout the retina, causing full-thickness necrosis and blindness. Lesions may be single or multiple and unilateral or bilateral, affecting any part of the retina. Symptoms include visual field loss, decreased visual acuity, floaters, and photopsias, but pain, redness, and photophobia are not features, and it is not uncommon for patients to be completely asymptomatic. The presence of subjective scotomata varies with the location of the lesions. CMV retinitis may appear as intraretinal yellow necrotic lesions with retinal hemorrhages (fulminant/edematous retinitis), as less densely necrotic without hemorrhage (indolent/granular retinitis), or some combination of the two (Fig. 16.2). Both types, however, are characterized by a dry-appearing, granular border. There is often a mild vitritis and anterior chamber inflammation. The disease is described according to the location within the retina—zone 1 is within 3000 μm of the center of the fovea or 1500 μm of the optic nerve; zone 2 is located from the periphery of zone 1 to the ampulla of the vortex veins; and zone 3 from the periphery of zone 2 to the ora serrata. Zone 1 lesions are generally considered immediately sight-threatening and usually require more urgent therapy than do more peripheral lesions. Causes of vision loss include central retinal necrosis, rhegmatogenous retinal detachment, secondary optic nerve involvement, or exudative swelling along an active border adjacent to the fovea.
As with other HIV-related infections, however, the most important treatment for CMV retinitis is immune recovery; all patients with CMV retinitis should be treated with ART unless there are medical contraindications or non-adherence to therapy is anticipated. All currently available therapy is virostatic, not virocidal, so patients who remain immunosuppressed require lifelong suppressive therapy, adding substantially to the cost and potential morbidity of treatment. Patients without immune recovery will on average get progression or breakthrough of retinitis, manifesting as new lesions or reactivation of a previously inactive lesion, within 2–3 months, even while continuing therapy. Development of ganciclovir resistance through UL97 or UL54 mutations is common within 6 months of therapy, but limited intraocular penetration across the blood–retina barrier with systemic therapy likely accounts for the initial episodes of recurrence. Antiviral resistance and breakthrough retinitis is much less common in ART-treated patients, but the increased mortality associated with the development of resistant CMV is of comparable magnitude in patients in the pre- and post-ART eras [16].
1. The incidence of CMV retinitis has fallen 80–90% [17].
2. Visual field loss is decreased six- to sevenfold in patients with immune recovery (CD4 count > 100 cells/mm3) [18].
3. Cessation of anti-CMV therapy is routinely possible in ART-treated patients who have maintained a CD4 count > 100 cells/mm3 for at least 3 months [17].
4. The risk of retinal detachment in eyes with CMV retinitis is decreased in patients taking ART [17].
5. Mortality is decreased in patients with CMV retinitis taking ART compared to those patients not on ART.
6. ART-experienced patients who develop CMV retinitis have more asymptomatic disease, better visual acuity in the better eye, more bilateral disease, and less zone 1 involvement compared to ART-naïve patients [19].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree