10.1 The two routes to ethical approval
There are two main routes for obtaining ethical approval: the National Research Ethics Service (NRES) and local university ethics committees. You must first establish whether you need to apply using the NRES or a local university system. If your project involves NHS property, staff or patients you need to use the NRES. Precise details of the remit of an NHS REC are given in Box 10.1; however, points (f) and (g) are open for review and may change. If you are a university based student or academic researcher, your project is based entirely outside the NHS and it involves only healthy human volunteers, you must use the local university system. In most cases the decision will be obvious but occasionally you may need further advice, in which case, ring your local REC officer who will be happy to help.
Ethical advice from the appropriate NHS research ethics committee (REC) is required for any research proposal involving:
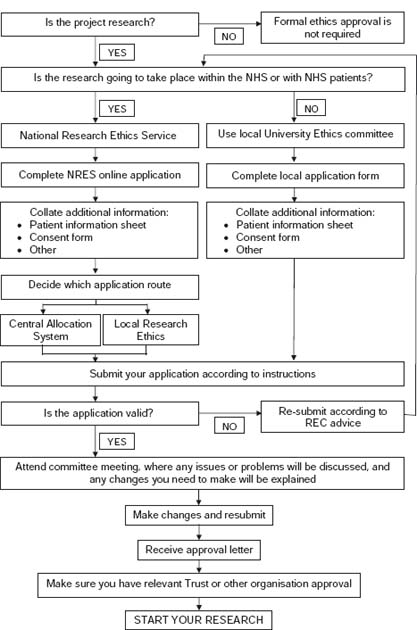
The majority of this chapter focuses on the NHS ethics system but the principles will be the same for university systems. I aim to outline the main stages of the process to guide you through your initial contact with a REC. Figure 10.1 is a flow chart showing the process you will need to go through and each key decision point.
10.1.1 An introduction to the National Research Ethics Service
In 2004 the process for obtaining ethical approval within the NHS was standardised and governance was taken over by the Central Office for Research Ethics Committees (COREC), which is now known as the National Research Ethics Service (NRES). At the time of writing this book there are major changes being piloted for the NHS ethics approval process and there are two which will potentially benefit you as a researcher: the ‘Early Provision of Advice’ and the ‘Fast Track of Studies Considered to Have no Material Ethical Issues’.
The pilot for ‘Early Provision of Advice’ was launched in May 2007 and is a screening process aiming to identify applications which are unlikely to meet the requirements of the REC, such as:
 Applications with poor presentation, which are incomplete, or have insufficient technical merit
Applications with poor presentation, which are incomplete, or have insufficient technical merit Studies submitted inappropriately (i.e. outside of the NHS REC’s remit)
Studies submitted inappropriately (i.e. outside of the NHS REC’s remit) Studies which may require further expert review in order for the REC to make a decision
Studies which may require further expert review in order for the REC to make a decisionThe NRES then provides advice to bring the application up to an appropriate standard suitable for REC review.
The second pilot for ‘Fast Track of Studies Considered to Have no Material Ethical Issues’ commenced in April 2007. This pilot hopes to develop a filter system where all applications are examined by a small trained review team. This team will be able to give fast track approval to those applications deemed to have no material ethical issues and thus do not require review by the full committee. The results of these pilots are expected in early 2008 and may result in alterations to the ethics process.
The evolving nature of the NRES means that it is important that you refer to the website (www.nres.npsa.nhs.uk) in order to obtain the most up to date advice on the application process.
10.1.2 An introduction to university ethics systems
Unlike the NHS there is not a uniform system for ethical approval throughout the academic community. Each university will have its own particular process, but all will follow the same basic requirements as the NHS system described in the rest of this chapter. The specific procedure and application form will vary so you must obtain local advice.
Figure 10.1 Flow chart of the ethical approval process.
10.2 The application form
The NRES use the same form for all studies and it must be filled in online, which requires registration at www.nresform.org.uk. Once registered, you can complete the form on any computer with access to the internet; you can save work as you go along and come back to partially completed forms at a later date. You can share the form with others to get their comments (as long as they are also registered), and duplicate the form to create a personalised template for future applications. The system also allows you to track your application and keep a record of all submissions made. The form is continually assessed and improved and the NRES are keen to encourage feedback from users to make the system as user friendly as possible. It has improved dramatically since the first version in 2004.
The first page of the NRES form is called the ‘form sieve’; the answers you give on this page will dictate which further answers you will need to give. Once you have completed this page the main form will appear with irrelevant questions automatically filtered out. There are two sections to the form; Section 1 is the main form and Section 2 is for site specific information (SSI). The REC will review all sections but Section 2 will also be used for local R & D approval of the research project. If you have more than one site you will need to complete an SSI for each site in the proposed research.
University RECs will have their own application form but the type of questions asked will be similar to the NHS version. Whichever form you are using, work through systematically, completing all the questions you can on your first pass. Make a note of those questions that need more work or for which you need to ask advice and come back to them later. If you have a well prepared research protocol, most of this will be a process of cutting and pasting. There is plenty of advice on the NRES website about each question as well as detailed guidance on the whole procedure. The following general navigational tips will help you use the online form:
 At the top and bottom of every page there is a row of buttons for navigation. The <navigate> button takes you to a list of all the questions so you can skip through the form.
At the top and bottom of every page there is a row of buttons for navigation. The <navigate> button takes you to a list of all the questions so you can skip through the form. Your work is automatically saved every time you move to the <next> or <previous> page or click <save now>.
Your work is automatically saved every time you move to the <next> or <previous> page or click <save now>. When typing long answers click the <save now> button frequently. Sometimes it is possible to lose work because the internet connection ‘times out’.
When typing long answers click the <save now> button frequently. Sometimes it is possible to lose work because the internet connection ‘times out’. It is usually better to type long answers into a word processor and then cut and paste into the online form.
It is usually better to type long answers into a word processor and then cut and paste into the online form. Next to every question is an <i> symbol for question-specific advice – check this if you are not sure how to answer a question. If you get stuck or are unsure of what information is needed, contact the ethics committee administrator, your R & D office or a local Research and Development Support Unit if you have one (see www.national-rdsu.org.uk).
Next to every question is an <i> symbol for question-specific advice – check this if you are not sure how to answer a question. If you get stuck or are unsure of what information is needed, contact the ethics committee administrator, your R & D office or a local Research and Development Support Unit if you have one (see www.national-rdsu.org.uk).10.3 Confidentiality
Confidentiality is a big issue and you will need to include information about how your data will be kept confidential in your ethics application. You will also need to include statements about confidentiality in the patient information sheet. Advice on suitable wording is provided on the NRES website. The ethics requirements are designed to ensure you do not breach the Data Protection Act 1998, which is a set of rules to limit the use of personal information relating to living people.
Your research notes should be kept in a secure location such as a locked department or locked cupboard or filing cabinet. If you are unable to use a secure environment to store your notes with identifying patient details, such as consent form, letters with name, address or hospital number, should be kept separately to the clinical data collection forms, so that the data cannot be linked to the patient.
When creating your database for the analysis do not use the patient’s name or other identifying information. Instead assign each patient a unique study number and use only this. Even though Hospital IT systems are designed to be secure and suitable to contain patient information, it is best practice to use only anonymous data.
It is customary to write to the patient’s GP or consultant to say they are on a trial and to provide relevant information; indeed ethics committees expect you to do this. Make sure this is stated in the information sheet and the consent form, so the patient knows you are going to share their information in this way.
10.4 What else do I have to submit with the main form?
Along with the full application form you will almost certainly have to submit a patient information sheet and consent form. As a general rule written informed consent must be obtained from all research participants. Only in special circumstances are you likely to run research without written consent. To obtain informed consent you will need both a patient information sheet to explain your research and a consent form for the patient to sign. In addition you may need to submit other information to support and clarify your application.
10.4.1 Participants’ information sheet
The participants’ information sheet tells the participants what you are going to do, why you are doing it, and what you expect of them. Once you have completed the application form there is a temptation to sigh with relief and quickly put together the participant information. DON’T – this is a very important document that you must get right.
Give yourself plenty of time to do this carefully. Often all the queries and requests for changes from the REC relate to the information provided for participants. There are specific guidelines provided for writing this, since it must contain prescribed information. It will be sent back for changes if all compulsory information is not included. Comprehensive guidelines are given on the NRES website and even if you are using a university REC, these guidelines will be helpful to you.
Ask other researchers for examples of information sheets if you are not sure how to proceed. It can be particularly useful to use the wording from an information sheet that has already been approved, for example when describing the process for taking blood, using genetics information and so on.
10.4.2 Participants’ consent form
The consent form provides the written evidence that you have gained the participants’ explicit informed consent to include them in the research study. This makes it a very important document so I have devoted the whole of Chapter 11 to examining the issue of participant consent.
The NRES consent form also has a standard format and a template is provided on the website. An example is given in Figure 10.2. University RECs also frequently provide a standard template to use. Make sure all the points listed in the standard form are applicable to your research and consider if there is anything else that you need to specifically highlight to your participants, for example, if you intend to tape interviews and retain the tape for further analysis, or if you will retain blood samples for future analysis.
10.4.3 Other supporting information
You will also be required to submit other information to support your application. This will include, as a minimum, a copy of your research protocol and your CV. If you are a student, your supervisor will also need to submit their CV. Other documents that are commonly required include: copies of letters you intend to send to participants or intended participants; copies of any recruitment posters or adverts you wish to use; copies of letters you will send to the patient’s GP or consultant to inform them of this patient’s participation; and any questionnaires or interview schedules that you intend to use.
Figure 10.2 Example of a consent form using the National Research Ethics Service template.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


