(Sandostatin, Sandostatin LAR) Do not confuse Sandostatin with Sandimmune, Sandostatin LAR, sargramostim, or simvastatin. Do not confuse ofatumumab with omalizumab. ◀ ALERT ▶ Do not give by IV push or bolus. Use in-line filter supplied with product. (Apo-Oflox BLACK BOX ALERT May increase risk of tendonitis, tendon rupture. Do not confuse Ocuflox with Ocufen. (Apo-Olanzapine Do not confuse olanzapine with olsalazine or quetiapine, or Zyprexa with Celexa or Zyrtec. Do not confuse Benicar with Mevacor. Obtain B/P, apical pulse immediately before each dose in addition to regular monitoring (be alert to fluctuations). If excessive reduction in B/P occurs, place pt in supine position, feet slightly elevated. Question for possibility of pregnancy (see Pregnancy Category). Assess medication history (esp. diuretics).
O
octreotide
ofatumumab
Adminstration/handling
![]() IV
IV
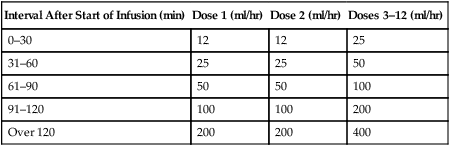
Interval After Start of Infusion (min)
Dose 1 (ml/hr)
Dose 2 (ml/hr)
Doses 3–12 (ml/hr)
0–30
12
12
25
31–60
25
25
50
61–90
50
50
100
91–120
100
100
200
Over 120
200
200
400
ofloxacin
![]() , Floxin Otic, Novo-Ofloxacin
, Floxin Otic, Novo-Ofloxacin ![]() , Ocuflox)
, Ocuflox)
olanzapine
![]()
![]() , Zyprexa, Zyprexa Intramuscular, Zyprexa Relprevv, Zyprexa Zydis)
, Zyprexa, Zyprexa Intramuscular, Zyprexa Relprevv, Zyprexa Zydis)
olmesartan
![]()
Nursing considerations
Baseline assessment
Nurse Key
Fastest Nurse Insight Engine
Get Clinical Tree app for offline access

 classification
classification classification
classification
 classification
classification classification
classification classification
classification classification
classification classification
classification classification
classification classification
classification

