Chapter 21 Nutritional foundation for pregnancy, childbirth and lactation
Learning outcomes for this chapter are:
1. To recognise the importance of weight gain during pregnancy and the use of body mass index for underweight and overweight women
2. To discuss the role and dietary sources of macronutrients and key micronutrients in pregnancy and lactation, and the special nutritional needs of pregnant teenagers and multiple pregnancies
3. To explain how diet can help alleviate some of the common conditions experienced in pregnancy, such as anaemia, nausea and vomiting, constipation and gastro-oesophageal reflux
4. To explain the role of nutrition in gestational diabetes mellitus and maternal obesity
5. To explain the risk of food-borne illness in pregnancy and to recognise the importance of food safety in pregnancy
6. To emphasise the importance of hydration and nourishment for childbirth and to recognise appropriate foods and drinks to consume in childbirth
7. To discuss the role of energy intake and output and their effects on lactation
8. To explain the role and limitations of allergy prevention during lactation.
This chapter outlines the importance of good nutrition during pregnancy in order to promote the growth and development of the fetus and maternal tissues, and to support the demands of childbirth and lactation. Dietary strategies are found to help alleviate some of the conditions associated with pregnancy and lactation, such as constipation, gastro-oesophageal reflux, nausea, maternal obesity, gestational diabetes, food-borne illness and allergy prevention. The chapter is intended to provide midwives with an understanding of the role of nutrients in pregnancy, childbirth and lactation, to identify appropriate dietary sources of these nutrients, and to present practical dietary strategies during pregnancy and lactation.
WEIGHT GAIN
Weight gain recommendations for pregnancy are based on women’s pre-pregnancy weight. The body mass index (BMI) is typically used as an indicator of body fat and, although this measurement is not valid during pregnancy, a woman’s pre-pregnancy BMI can be used to recommend total weight gain during pregnancy (Table 21.1).
Table 21.1 Recommended weight gain during pregnancy based on pre-pregnancy BMI
| Pre-pregnancy BMI | Recommended weight gain (kg) | Rates of weight gain∗ 2nd and 3rd trimester |
|---|---|---|
| Underweight: < 18.5 kg/m2 | 12.5–18 | 0.51 kg (0.44–0.58) |
| Healthy: 18.5–24.9 kg/m2 | 11.5–16 | 0.42 kg (0.35–0.50) |
| Overweight: 25–29.9 kg/m2 | 7.0–11.5 | 0.28 kg (0.23–0.33) |
| Obese: ≥30.0 kg/m2 | 5–9 | 0.22 kg (0.17–0.27) |
BMI (body mass index) is calculated as weight (kg) divided by height squared (m2).
∗ Calculations assume a 0.5–2 kg weight gain in the first trimester.
(Source: National Academy of Sciences 2009)
For women in the normal weight BMI range, an average weight gain of 11.5–16 kg is recommended. Women in the normal weight BMI range tend to gain an appropriate amount of weight during pregnancy. However, women who are underweight pre-pregnancy have increased rates of pregnancy loss and small-for-gestational-age or low-birthweight infants. It has been suggested that these risks are simply due to a lack of maternal energy stores (Gluckman et al 2008). Therefore, weight gain for these women tends to be higher than for women with healthy pre-pregnancy BMI (12.5–18 kg). Women who are overweight pre-pregnancy are at increased risk of developing gestational diabetes and hypertensive disorders or undergoing caesarean section, with the accompanying anaesthetic and postoperative risk (Sarwer et al 2006). The recommended weight gain for women with a BMI of 25–29.9 kg/m2 is 7–11.5 kg; and this further decreases for those with a BMI of ≥30.0 kg/m2 to 5–9 kg (refer to the section on maternal obesity, later in the chapter). The BMI guidelines have recently been reviewed (National Academy of Sciences 2009) and the recommendations for obese women included, due to the increasing rates of obesity in Australia (National Preventative Health Taskforce 2009) and New Zealand (Ministry of Health 2007).
MACRONUTRIENTS
Fats
Linoleic acid, an omega-6 fat, and alpha-linolenic acid, an omega-3 fat, are essential fats required for growth and development. These fats cannot be synthesised by the body and therefore must be consumed in our diet from foods (Table 21.2).
Table 21.2 Dietary sources of essential and long-chain polyunsaturated fats
| Fat | Dietary sources |
|---|---|
| Essential fats | |
| Linoleic acid (LA) | Seeds and nuts, vegetable oils, soft margarines |
| Alpha-linolenic acid (LNA) | Canola and flaxseed oil, walnuts, peanuts, pecans |
| Long-chain polyunsaturated fats | |
| Arachidonic acid (AA) | Meat, eggs, milk, cheese, yoghurt |
| Eicosapentaenoic acid (EPA) | Fatty fish (salmon, tuna, mackerel, herring, sardines) |
| Docosahexaenoic acid (DHA) | Fatty fish (salmon, tuna, mackerel, herring, sardines) |
Each of the essential fats is responsible for producing one or more specific LCPs (Fig 21.1). Two of the LCPs, specifically arachidonic acid (AA) and eicosapentaenoic acid (EPA), are precursors to a group of hormone-like compounds called eicosanoids. Eicosanoids have an important role in maintaining blood pressure and haemostasis, in inflammation and in parturition. The eicosanoids produced from AA promote vasoconstriction, platelet aggregation and inflammation, whereas the eicosanoids produced from EPA promote vasodilation, inhibit platelet aggregation and reduce inflammation. During parturition, the eicosanoids produced from AA appear to enhance the breakdown of collagen fibres within the cervix, whereas the eicosanoids produced from EPA tend to inhibit this process (Allen & Harris 2001).
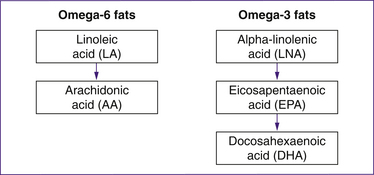
Figure 21.1 Conversion of the essential fats into long-chain polyunsaturated fats
(based on Allen & Harris 2001)
In Northern European countries, where the intake of fatty fish (and therefore EPA) is high, the incidence of preterm birth is low. This has sparked considerable research into the effect of EPA on parturition, specifically in women at risk of preterm labour. The results of these studies suggest that supplementation with EPA or consumption of fish 2–3 times per week may reduce the risk of preterm labour in some groups of women (Cetin & Koletzko 2008; Makrides 2008).
One concern that arises with recommendations to increase fish consumption during pregnancy is the amount of mercury present in some fish species. Larger fish tend to accumulate mercury. Food Standards Australia New Zealand (FSANZ) has specific recommendations regarding fish consumption, mercury levels and pregnancy (Table 21.3) (FSANZ 2004).
Table 21.3 Recommendations for fish intake during pregnancy
| Country | Recommended intake |
|---|---|
| Australia1 | |
| New Zealand2 | Consume fish but limit intake of the following species to no more than 1–2 servings per week (1 serve = 150 g): school shark, swordfish, southern bluefin tuna, marlin, North Island lake trout, dogfish, cardinal fish |
1. Source: Food Standards Australia New Zealand 2004.
Carbohydrates
Although carbohydrates should account for the majority of energy intake during pregnancy, the type of carbohydrate consumed is also important to prevent large fluctuations in blood glucose levels. The rate at which dietary carbohydrate, specifically glucose, is absorbed into the bloodstream can be measured, and is referred to as the glycaemic index (Table 21.4). Some carbohydrates present in food are rapidly digested in the digestive tract, thereby entering circulation quickly. This can result in a large increase in blood glucose followed by a sudden drop in blood glucose. Foods containing these types of carbohydrates are referred to as having a high glycaemic index value. In contrast, some carbohydrates are digested more slowly, allowing for a slow and steady rise in blood glucose that is not followed by a sudden drop in blood glucose levels. Foods containing these types of carbohydrates are referred to as having a low glycaemic index value. For sustained energy, foods with a low glycaemic index value are recommended.
Protein
During pregnancy the requirement for dietary protein is slightly higher than in pre-pregnancy, to account for the development of maternal and fetal tissues. The protein requirements are based on bodyweight; for every kilogram of bodyweight a non-pregnant woman should consume between 0.8 and 1.0 g of protein, approximately 60 g per day (NHMRC 2006).
Despite limited evidence, high-protein diets have been suggested to help minimise the risk of preeclampsia. The World Health Organization does not support these diets and clearly states that in the absence of a protein deficiency, protein supplementation is unlikely to reduce a woman’s risk of developing preeclampsia (Kramer & Kakuma 2004). The current intakes of protein in both Australia and New Zealand are sufficient to meet the increased requirements during pregnancy, and therefore for most supplementation is not necessary (ABS 1995; Ministry of Health 1999).
MICRONUTRIENTS: MINERALS
There are many minerals that the human body requires to perform a variety of important functions. Only those with a significant role during pregnancy will be highlighted in this chapter.
Iron
Iron is an important mineral present within the haem structure of haemoglobin. In any anabolic condition, such as pregnancy, the increased demands for haemoglobin result in increased demands for iron. During pregnancy, as well as in childhood, intestinal absorption of iron increases to help meet these demands. Despite enhanced absorption during pregnancy, iron deficiency and iron deficiency anaemia still occur in many pregnant women. Iron deficiency is particularly prevalent in indigenous populations—among Māori 40% (Ministry of Health 1999) and among Aboriginal Australians 55% (WHO 2007) are iron deficient—and is also a problem for immigrant populations.
Iron is present in food (Table 21.5) in two forms, haem and non-haem iron. Haem iron is the form present in meat, poultry and fish, as a component of haemoglobin. Non-haem iron is also present in meat, poultry and fish but is the only form found in plant foods, fortified foods and supplements.
Although iron is present in many foods, not all the iron is absorbed and used by the body. Haem iron is more readily absorbed in the intestine than non-haem iron. Up to one-third of haem iron is absorbed, whereas the absorption of non-haem iron is usually less than 10% (Zimmerman & Hurrell 2007). Including foods rich in vitamin C increases the absorption of non-haem iron by keeping iron in a more readily absorbable form—ferrous iron. Consumption of meat, poultry or fish together with non-haem iron also enhances iron absorption.
In addition to these enhancers or promoters of iron absorption, there are several factors that inhibit iron absorption. Tannins, present in black teas and red wine, have been reported to reduce iron absorption by up to 40%; therefore, it is recommended that these beverages be consumed two to three hours before or after a meal. Another inhibitor of iron absorption is phytates, phosphate-containing molecules present in legumes, nuts and whole grains that bind iron, rendering it unavailable for absorption. Soaking legumes, roasting nuts and fermenting whole grains decreases the phytate content of these foods, thereby decreasing their effect on iron absorption (Hallberg 2002). Finally, high doses of calcium (300 mg) or zinc (15 mg), such as the level present in supplements, consumed with iron-rich foods or iron supplements have been reported to inhibit iron absorption (Zimmerman & Hurrell 2007).
Calcium
Calcium is the most prevalent mineral in our body, with most of it contained within our skeleton. In addition to its structural role, it is involved in neural transmission, haemostasis and muscular contraction. Calcium may also play a role in regulating blood pressure. A Cochrane review of calcium supplementation and its role in preventing hypertensive disorders and problems found that in studies where women were high risk or had a low baseline calcium intake, supplementing with at least 1 g of calcium daily resulted in a significant reduction in pre-eclampsia (Hofmeyr et al 2006).
Calcium is present in many animal and plant foods (Table 21.5). As with iron, the bioavailability of calcium is much higher in animal foods such as milk, yoghurt and cheese than in plant foods. The calcium present in plant foods may also interact with various inhibitors, including phytates, high doses of minerals such as iron, and oxalates. Oxalates are compounds present in foods such as spinach, rhubarb and chocolate. Like phytates, they bind calcium, rendering it unavailable for absorption in the intestinal tract.
Table 21.5 Dietary sources of iron and calcium
| Mineral | Animal sources | Plant sources |
|---|---|---|
| Iron | ||
| Calcium |
Zinc
Zinc is an important component of over 80 enzymes involved in many reactions, including cell replication and growth, regulation and expression of genes, optimal immune function and hormone activity. Findings from several observational studies suggest that zinc deficiency during pregnancy increases the incidence of neural tube defects, fetal death and malformations, low birthweight, premature rupture of membranes and increased antenatal and intrapartum complications. There have been a number of randomised controlled trials of zinc supplementation during pregnancy; only half of these have reported an effect in reducing adverse birth outcomes or complications. It has been suggested that zinc supplementation is beneficial in developing countries where zinc deficiency is widespread (Cole & Lifshitz 2008).
MICRONUTRIENTS: VITAMINS
Folate
Folate is a generic term used to describe a collection of compounds, of which folic acid is the most stable and absorbable form. Folate is involved in cell replication and division, maturation of erythrocytes and amino acid metabolism. Folic acid also reduces the incidence of neonatal neural tube defects when taken prior to and following conception. Folate insufficiency has also been associated with cleft lip, cleft palate, placental abruption, low birthweight, miscarriage and Down syndrome. The recommendations for folate intake in women of childbearing age are the same for Australia and New Zealand (Table 21.6).
Table 21.6 Folate recommendations for Australian and New Zealand women
| Australia | New Zealand |
|---|---|
| All women planning a pregnancy should supplement their diets with 400 μg of folic acid per day beginning one month prior to conception until 12 weeks gestation. | All women planning a pregnancy should supplement their diets with 400 μg of folic acid per day beginning one month prior to conception until 12 weeks gestation. |
| Women with a previous neural-tube-defect-affected pregnancy or with close family history of neural-tube-defect-affected pregnancy should supplement their diet with 5 mg of folic acid/day. | |
(Sources: NHMRC 2006; Ministry of Health 2006)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree