CHAPTER 5 Nursing patients with endocrine and metabolic disorders
Part 1 Nursing patients with endocrine disorders
Introduction
Endocrinology is a rapidly growing field (Wilson 2005). Considerable progress is being made, with important implications for other areas of medicine, e.g. neuroendocrinology, which in turn has important applications within psychiatry. Advances are constantly being made and current research is adding to knowledge of genetic causes of many endocrine conditions. Genetic research is an area from which future treatments of endocrine disease will come.
Apart from diabetes mellitus (see pp. 149–175) and thyroid disorders, endocrine problems are not common. Many endocrine disorders are rarely seen and may be difficult to diagnose without complicated and exhaustive testing. As a result, patients may be referred by their general practitioner or local hospital to specialist centres for diagnosis. For the patient, this has the advantage of offering highly specialised care. It also means that relatively few nurses have the opportunity to care for people with these disorders. However, given the wide-ranging effects of endocrine dysfunction, it is important that all nurses have a general understanding of endocrinology. Endocrine diseases can affect every system of the body, sometimes causing disfigurement and a change in body image and quality of life (QoL) and even occasionally posing a threat to life. These disorders can seriously affect the patient’s psychological outlook, either as a direct result of the illness or by virtue of the individual’s reaction to it.
As the tests and investigations required to diagnose some of the rarer endocrine conditions are often long and exhausting, clear explanations are essential so that the patient understands the need for them and the procedures that will be followed. This will help to reduce anxiety and increase the patient’s confidence in the health care team. The psychological support that the nurse can offer this group of patients is vitally important. Many benefit from referral to a support group (see Useful websites) and the contact this brings with other patients who have the same condition. Often these patients are young and probably otherwise in good health, and therefore the impact of endocrine disease on their body image and/or self-esteem should not be underestimated. For example, patients experiencing sexual dysfunction may feel embarrassed to talk to and seek support from family members and friends –in such cases, support from the health care professional is crucial.
Anatomy and physiology
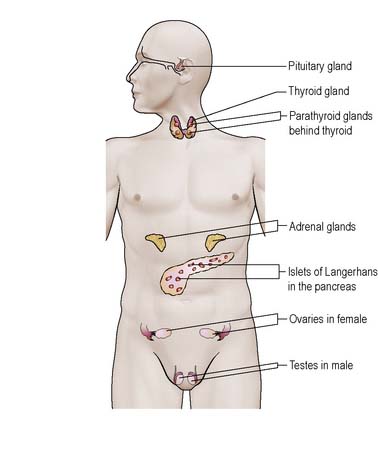
The nature of the endocrine system, being one of control, means that it has far-reaching effects throughout the body on various target organs. This section provides a brief outline of hormonal action and outlines the anatomy and physiology of individual endocrine glands and structures (see Further reading, e.g. Montague et al 2005). The gonads, ovaries and testes are described in Chapters 7 and 8.
Hormones
The endocrine system is one of the two major control systems of the body, the other being the nervous system. The nervous system mediates endocrine activity. The endocrine glands/structures produce hormones, which are secreted into the bloodstream for transport to their respective target organs (Figure 5.1). Hormones are either lipid-based (e.g. glucocorticoids) or peptides (e.g. adrenaline [epinephrine], insulin).
Control
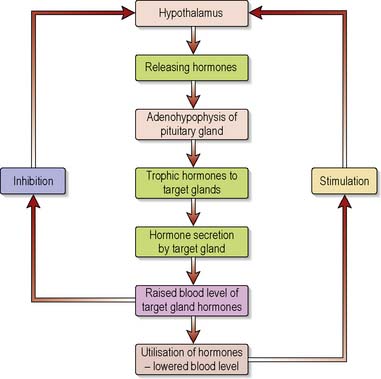
Most hormone systems are controlled by a negative feedback system which ensures that hormone levels, whilst fluctuating, remain within a preset range (see Appendix – normal values). Figure 5.2 illustrates how negative feedback operates in the hypothalamic–pituitary–thyroid axis.
Pituitary
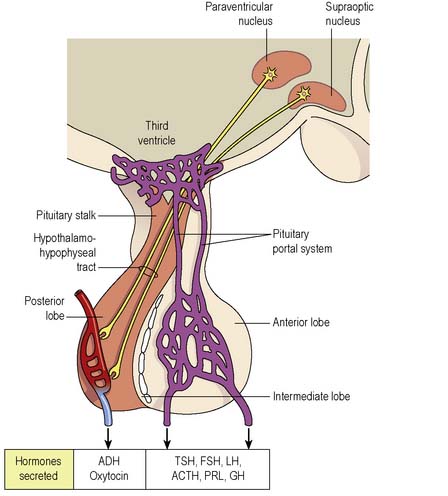
The pituitary gland lies immediately below the hypothalamus (part of the floor of the third ventricle of the brain) in the pituitary fossa. It is connected to the hypothalamus by the pituitary stalk and comprises two lobes which function independently of each other (Figure 5.3). During fetal life the glandular anterior lobe develops from the oropharynx to become the adenohypophysis. The neural posterior lobe is a down-growth from the forebrain, which becomes the neurohypophysis. The posterior pituitary receives nerve fibres from hypothalamic nuclei via the hypothalamohypophyseal tract in the pituitary stalk.
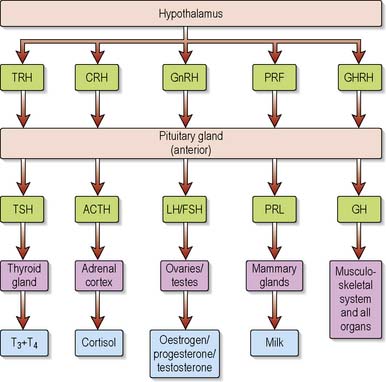
The hypothalamus contains specialised cells or nuclei (paraventricular and supraoptic) which synthesise the hormones antidiuretic hormone (ADH) (also known as arginine vasopressin [AVP] or vasopressin) and oxytocin. The hypothalamus also produces releasing/inhibiting hormones that regulate the secretion of anterior pituitary hormones (Figure 5.4). The hypothalamus also controls many centres for functions such as appetite, thirst, temperature regulation, sexual activity and sleep/wake cycles.
The pituitary stalk carries blood to both lobes of the pituitary in a portal system by which the hypothalamic releasing/inhibitory hormones are carried to the anterior pituitary. The trophic hormones produced in the anterior pituitary stimulate the other endocrine glands. ADH and oxytocin are stored in the posterior pituitary until released. Table 5.1 outlines the hormones secreted by the pituitary gland.
Table 5.1 Hormones of the pituitary gland and their action
| Hormone | Action |
|---|---|
| Anterior lobe | |
| Growth hormone (GH) (syn. somatotrophin) | Pulsatile release. Does not have a single target gland but acts on a variety of tissues, e.g. bone, viscera and soft tissues. It is particularly important in children to stimulate growth. Action in adults recently thought to involve maintenance of cardiovascular activity, fat deposition and muscle development |
| Prolactin (PRL) | Main action is to stimulate lactation in females; also stimulates the corpus luteum of the ovary to secrete progesterone |
| Adrenocorticotrophic hormone (ACTH) | Secreted under circadian rhythm. Stimulates the adrenal cortex to secrete corticosteroids |
| Luteinising hormone (LH) known as interstitial cell-stimulating hormone (ICSH) in men (gonadotrophins) | In women LH promotes ovulation; stimulates formation of the corpus luteum. In men ICSH stimulates the production of the androgen hormone testosterone |
| Follicle-stimulating hormone (FSH) (a gonadotrophin) | FSH stimulates the production of sex hormones and contributes to regulation of the menstrual cycle in women. In women, stimulates the production of ovarian follicles and secretion of oestrogen; in men, stimulates spermatogenesis |
| Thyroid-stimulating hormone (TSH) | Stimulates the thyroid to release the hormone thyroxine |
| Posterior lobe | |
|---|---|
| Antidiuretic hormone (ADH) (also known as arginine vasopressin [AVP] or vasopressin) | Controls water homeostasis in the body by regulating water reabsorption from the distal convoluted tubules of the kidney |
| Oxytocin | Induces uterine contraction during labour (unusually controlled by a positive feedback mechanism) and ejection of milk from breasts postpartum |
Thyroid
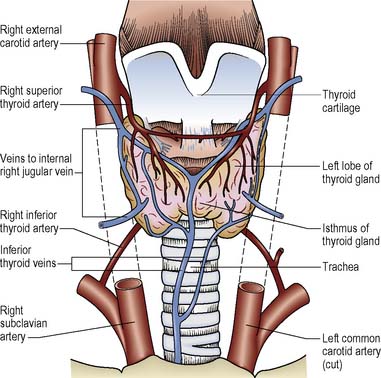
The thyroid is a gland in the neck sited anteriorly to the larynx and attached to the thyroid cartilages and the trachea. It comprises two lobes connected by an isthmus (Figure 5.5). Structures in close proximity include the oesophagus, the parathyroid glands, the recurrent laryngeal nerves and the carotid arteries.
The hormones, thyroxine (T4) and some circulating tri-iodothyronine (T3), are synthesised and secreted by the thyroid gland. Thyroid hormone production is dependent on the availability of iodine in the diet (see Ch. 21). More T4 than T3 is produced, but T4 is converted in some tissues to the more biologically active T3. Over 99% of all thyroid hormone is bound to plasma thyroid-binding proteins. Only the free hormone is available for use by the tissues. If the levels of T4 and T3 in the blood fall, the hypothalamus releases thyrotrophin-releasing hormone (TRH). As the levels of TRH rise, the pituitary gland secretes thyroid-stimulating hormone (TSH) which stimulates the thyroid gland to produce more T4 and T3. The principal effect of thyroid hormones is to influence the metabolism of cells and, therefore, the metabolic rate. Low levels of hormone are associated with a low temperature, i.e. patients will complain of feeling cold, slow heart rate, weight gain, poor concentration and low mood whereas patients with high concentrations of thyroid hormones experience a rapid heart rate, heat intolerance, anxiety and weight loss despite increased appetite.
Parathyroids
Vitamin D and calcitonin are also involved in calcium metabolism (Box 5.1).
Adrenals
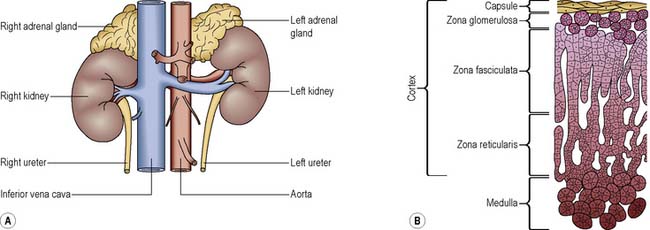
The adrenal glands are situated one on the upper part of each kidney (Figure 5.6A). They are highly vascular and derive their blood supply from the renal arteries, the inferior phrenic arteries and directly from the aorta; they are drained by the suprarenal veins.
Adrenal cortex
The adrenal cortex has three layers (Figure 5.6B) that produce different hormones, collectively termed corticosteroids:
Mineralocorticoids
The most important of the mineralocorticoids is aldosterone, which is the most potent regulator of sodium and potassium and is important in maintaining acid–base balance. Aldosterone acts on the distal convoluted tubules of the kidney and stimulates the cells to reabsorb and thus conserve sodium (see Chs 8, 20).
Adrenal medulla
In normal secretion, adrenaline probably has some effect on mean blood pressure; although it increases systolic pressure, it decreases diastolic pressure. When secreted under the control of the sympathetic nervous system (Ch. 9) it causes tachycardia, decreased gut motility and the closure of sphincters in the gut, pupil dilatation, bronchodilatation and piloerection.
Noradrenaline raises both systolic and diastolic blood pressure.
Disorders of the pituitary and hypothalamus
Hypersecretion of a hormone usually arises from a so-called ‘functioning’ pituitary adenoma. As this adenoma grows, it is likely to disrupt the normal function of other areas of the pituitary, and as a result progressive hypopituitarism occurs. This failure of the pituitary hormones occurs in a characteristic sequence (Boon et al 2006) – first GH is lost, followed by the gonadotrophins LH/ICSH and FSH, ACTH and then TSH. PRL deficiency is rare except as seen postpartum in Sheehan’s syndrome.
Hypersecretion of anterior pituitary hormones
Cushing’s disease caused by an excess of glucocorticoid hormones stimulated by an ACTH-producing adenoma of the pituitary is discussed in a later section (see p. 142).
Hypersecretion of growth hormone: acromegaly
Acromegaly is a rare condition caused by prolonged, excessive secretion of GH from a benign tumour of the pituitary gland occurring after fusion of the epiphyses of the long bones. It affects both sexes equally. The mean age at diagnosis is between 40 and 50 years, although excessive secretion of GH can occur at any age. In children where epiphyseal fusion has not yet occurred, it leads to gigantism. Acromegaly leads to a reduction in life expectancy with a two- to four-fold increase in mortality rate (Colao et al 2004).
Pathophysiology
Medical management
Investigations
Diagnosis can be confirmed following clinical examination and pituitary function testing. On clinical examination, some or all of the symptoms listed above may be present. Magnetic resonance imaging (MRI) usually shows the pituitary tumour and whether there is enlargement of the pituitary fossa. If acromegaly is suspected, a blood sample should be obtained and an insulin-like growth factor 1 (IGF-1) and random GH level measured. If the IGF-1 level is ‘normal’, when compared to the age-adjusted range, and GH <0.5 mU/L, the diagnosis of acromegaly can be excluded (Trainer 2002). Otherwise, the patient should proceed to have an oral glucose tolerance test (OGTT). The OGTT has been the gold standard for the diagnosis of acromegaly for many years. False positives may occur if the patient has diabetes mellitus, renal or liver disease, or anorexia nervosa. In acromegaly, measurement of GH levels during the OGTT are consistently elevated and fail to suppress to <2 mU/L in response to a 75 g oral glucose load. Normally, the level of GH would be undetectable. Acromegaly is therefore diagnosed on the basis of an elevated IGF-1 and failure to suppress GH during the OGTT. Further pituitary function tests may be performed to assess whether the remainder of the pituitary gland has been compromised. Prolactin is co-secreted in 30% of tumours.
Treatment
In untreated acromegaly, the mortality is nearly twice that of the normal population; early treatment is therefore essential. The aim is to relieve and, if possible, resolve symptoms associated with elevated GH and IGF-1 levels and the local effects caused by the tumour mass itself, and to prevent co-morbidity by lowering mean GH and IGF-1 levels to accepted ‘safe’ levels with minimal disruption to the patient’s lifestyle. Treatment may take the form of surgery, radiotherapy or medication. Until recently, surgery and radiotherapy were used in conjunction, with medical therapy then used to control residual excess GH secretion, as radiotherapy only lowers GH levels gradually. Today, medical therapy may also be used pre-operatively to control symptoms or to attempt to shrink a large tumour prior to surgery (Ben-Shlomo & Melmed, 2008). In patients unfit for surgery, medical therapy alone may be used.
Surgery
is currently regarded as the first-line therapy for acromegaly and, when performed by a pituitary surgeon, remains the treatment of choice. Hypophysectomy via the trans-sphenoidal route, which has a low morbidity and mortality, is the surgical procedure most commonly used. It results in a rapid fall in GH levels, often with no loss of any other pituitary hormones. Surgery is both rapid and inexpensive when compared to medical therapies. In patients with microadenomas <1 cm diameter it is curative in ~80% of cases. With macroadenomas >1 cm diameter it is nearer 40% (Kaltsas et al 2001).
Radiotherapy
Conventional external beam radiotherapy (25 fractions over 5 weeks delivered daily Monday–Friday) results in a delayed fall in GH and IGF-1 levels over the following decade which is paralleled by the development of hypopituitarism. After 5 years, 63% of patients have a normal IGF-1 (Jenkins et al 2006). Although used less often nowadays, it is safe, and radiation-induced visual damage is extremely rare. Patients may feel tired during the period of treatment and may experience some temporary hair loss at the site of entry of the beams. It is a useful treatment for patients in whom surgery is contraindicated or if GH/IGF-1 levels are not too high and/or there is a large tumour or residual tumour postoperatively, or where medical therapy fails to control GH/IGF-1 levels. Another form of radiotherapy is stereotactic radiotherapy, which may be single fraction radiosurgery or linear accelerator based stereotactic radiotherapy (multiple fraction). The expense is greater than conventional radiotherapy but is a ‘one-off’ cost. Long-term data are required, but early results suggest that GH and IGF-1 levels fall more rapidly after radiosurgery than with conventional radiotherapy and there is less damage to surrounding tissues, which may reduce the incidence of radiotherapy-induced hypopituitarism (Swords et al 2003). These two forms of radiotherapy can be given following conventional radiotherapy but cannot be used if the tumour is close to the optic chiasm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree