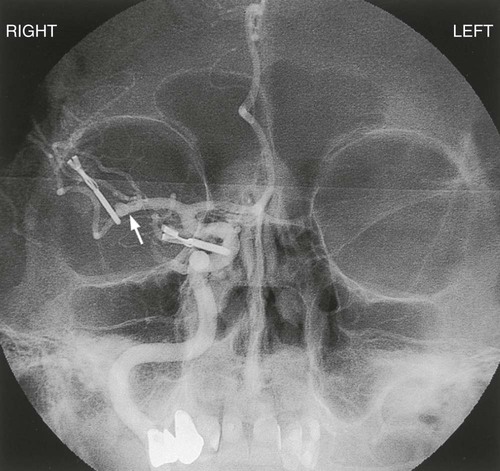
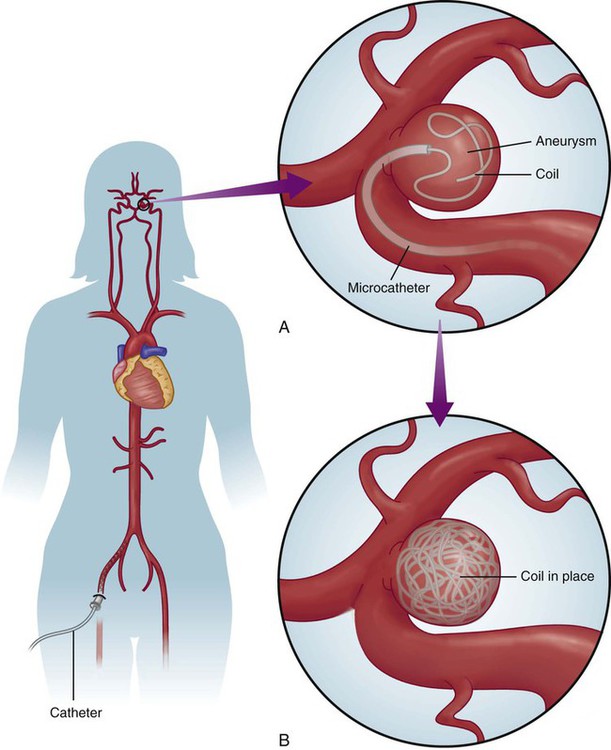
Chapter 24 Lourdes Januszewicz and Barbara Buesch Normal consciousness requires awareness and arousal. Awareness is the combination of cognition (mental and intellectual) and affect (mood) that can be construed based on the patient’s interaction with the environment.1 Alterations of consciousness may be the result of deficits in awareness, arousal, or both.2 The four discrete disorders of consciousness are: 1) coma, 2) vegetative state, 3) minimally conscious state, and 4) locked-in syndrome. Coma is characterized by the absence of both wakefulness and awareness, whereas a vegetative state is characterized by the presence of wakefulness with the absence of awareness. In the minimally conscious state, wakefulness is present, and awareness is severely diminished but not absent. Locked-in syndrome is characterized by the presence of wakefulness and awareness, but with quadriplegia and the inability to communicate verbally; thus, the patient appears to be unconscious.3 Box 24-1 lists the disorders of consciousness in descending order of wakefulness. Coma is the deepest state of unconsciousness; arousal and awareness are lacking.1–3 The patient cannot be aroused and does not demonstrate any purposeful response to the surrounding environment.4 Coma is a symptom rather than a disease, and it occurs as a result of some underlying process.1,2 The incidence of coma is difficult to ascertain because a wide variety of conditions can induce coma.1,2 This state of unconsciousness is, unfortunately, very commonly encountered in the critical care unit, and it is the focus of the following discussion. The causes of coma can be divided into two general categories: 1) structural or surgical and 2) metabolic or medical. Structural causes of coma include ischemic stroke, intracerebral hemorrhage (ICH), trauma, and brain tumors.5 Metabolic causes of coma include drug overdose, infectious diseases, endocrine disorders, and poisonings.5 Coma demands immediate attention, resulting in a high percentage of admissions to all hospital services.6 Box 24-2 provides a list of the possible causes of coma. Consciousness involves arousal, or wakefulness, and awareness. Neither of these functions is present in the patient in coma. Ascending fibers of the reticular activating system (ARAS) in the pons, hypothalamus, and thalamus maintain arousal as an autonomic function. Neurons in the cerebral cortex are responsible for awareness. Diffuse dysfunction of both cerebral hemispheres and diffuse or focal dysfunction of the reticular activating system can produce coma.1,6,7 Structural causes usually produce compression or dysfunction in the area of the ARAS, whereas most medical causes lead to general dysfunction of both cerebral hemispheres.8 Trauma, hemorrhage, and tumor can damage the ARAS, leading to coma. Destruction of large regions of bilateral cerebral hemispheres can be the result of seizures or viral agents. Toxic drugs, toxins, or metabolic abnormalities can suppress cerebral function.5–7 The clinical diagnosis of the comatose state is readily established by assessment of the level of consciousness. However, determining the full nature and cause of coma requires a thorough history and physical examination. A medical history is essential because events immediately preceding the change in the level of consciousness can often provide valuable clues to the origin of the coma. When limited information is available and the coma is profound, the response of the patient to emergent treatment may provide clues to the underlying diagnosis; for example, the patient who becomes responsive with the administration of naloxone can be presumed to have ingested some type of opiate.6 Detailed serial neurologic examinations are essential for all patients in coma. Assessment of pupillary size and reaction to light (normal, sluggish, or fixed), extraocular eye movements (normal, asymmetric, or absent), motor response to pain (normal, decorticate, decerebrate, or flaccid), and breathing pattern yields important clues for determining whether the cause of coma is structural or metabolic.1,6 The areas of the brainstem that control consciousness and pupillary responses are anatomically adjacent. The sympathetic and parasympathetic nervous systems control pupillary dilation and constriction, respectively. The anatomic directions of these pathways are known, and changes in pupillary responses can help identify where a lesion may be located (see Fig. 23-4 in Chapter 23). For example, if damage occurs in the midbrain region, pupils will be slightly enlarged and unresponsive to light. Lesions that compress the third nerve result in a fixed and dilated pupil on the same side as the neurologic insult. Pupillary responses are usually preserved when the cause of coma is metabolic in origin. Pupillary light responses are often the key to differentiating between structural and metabolic causes of coma.1,6,7,9 Areas of the brainstem adjacent to those responsible for consciousness also control the oculomotor eye movement. The ability to maintain conjugate gaze requires preservation of the internuclear connections of cranial nerves III, VI, and VIII by means of the medial longitudinal fasciculus (MLF).8 As with pupillary responses, structural lesions that impinge on these pathways cause oculomotor dysfunction such as a disconjugate gaze. Deficits in extraocular eye movements usually accompany a structural cause.1,5,9 Focal or asymmetric motor deficits usually indicate structural lesions.1,5 Abnormal motor movements may also help pinpoint the location of a lesion. Decorticate posturing (abnormal flexion) can be seen with damage to the diencephalon. Decerebrate posturing (abnormal extension) can be seen with damage to the midbrain and pons. Flaccid posturing is an ominous sign and can be seen with damage to the medulla.9 Abnormal breathing patterns may also assist in differentiating structural from metabolic causes of coma. Cheyne-Stokes respirations are seen in patients with cerebral hemispheric dysfunction or metabolic suppression. Central neurogenic hyperventilation, or Kussmaul breathing, occurs with metabolic acidosis or damage to the midbrain and upper pons. Apneustic breathing may occur with damage to the pons, hypoglycemia, and anoxia. Ataxic breathing occurs with damage to the medulla. Agonal breathing occurs with failure of the respiratory centers in the medulla.6,9 In addition to physical assessment, laboratory studies and diagnostic procedures are done. Structural causes of coma are usually readily apparent with computed tomography (CT) or magnetic resonance imaging (MRI).4,8 Laboratory studies are also used to identify metabolic or endocrine abnormalities.7 Evoked potentials are also useful in facilitating a differential diagnosis between the disorders of consciousness and in evaluating a patient’s prognosis. Generally, a patient in coma, with absence of brainstem auditory evoked responses (BAERs), is considered to have a poor prognosis of recovery.3 Occasionally, the cause of coma is never clearly determined. The goal of medical management of the patient in coma is identification and treatment of the underlying cause of the condition. Initial medical management includes emergency measures to support vital functions and prevent further neurologic deterioration. Protection of the airway and ventilatory assistance are often needed. Administration of thiamine (at least 100 milligrams [mg]), glucose, and a opioid antagonist is suggested when the cause of coma is not immediately known.1,6 Thiamine is administered before glucose because the coma produced by thiamine deficiency, Wernicke encephalopathy, can be precipitated by a glucose load.1 The patient who remains in coma after emergent treatment requires supportive measures to maintain physiologic body functions and prevent complications. Intubation for continued airway protection and nutritional support are essential. Fluid and electrolyte management is often complex because of alterations in the neurohormonal system. Anticonvulsant therapy may be necessary to prevent further ischemic damage to the brain.1,5,6 The health care team and the patient’s family make decisions jointly regarding the level of medical management to be provided. Family members require informational support in terms of the probable cause of coma and the prognosis for recovery of consciousness and function. Prognosis depends on the cause of coma and the length of time unconsciousness persists. Only 15% of patients in nontraumatic coma make a satisfactory recovery.7 Metabolic coma usually has a better prognosis compared with coma caused by a structural lesion, and traumatic coma usually has a better outcome compared with nontraumatic coma.5,7 Much research has been directed toward identifying the prognostic indicators for the patient in coma after a cardiopulmonary arrest. In a meta-analysis, the best predictors of poor outcome after cardiac arrest were lack of corneal or papillary response at 24 hours and lack of motor movement at 72 hours. However, regardless of the cause or duration of coma, outcome for an individual cannot be predicted with 100% accuracy.10 Research has focused on induced hypothermia in patients after cardiac arrest. The use of hypothermia has demonstrated improved neurologic outcomes and survival rates. When a patient remains comatose after return of spontaneous circulation, the body is cooled to 32° C to 34° C for up to 24 hours.11,12 Nursing management of the patient in coma incorporates a variety of nursing diagnoses (Box 24-3) and is directed by the specific cause of the coma, although some common interventions are used. The patient in coma totally depends on the health care team. Nursing interventions focus on monitoring for changes in neurologic status and clues to the origin of the coma, supporting all body functions, maintaining surveillance for complications, providing comfort and emotional support, and initiating rehabilitation measures.1 Measures to support body functions include promoting pulmonary hygiene, maintaining skin integrity, initiating range-of-motion exercises, managing bowel and bladder functions, and ensuring adequate nutritional support.1 The blink reflex is often diminished or absent in the patient in coma. The eyelids may be flaccid and may depend on body positioning to remain in a closed position, and edema may prevent complete closure. Loss of these protective mechanisms results in drying and ulceration of the cornea, which can lead to permanent scarring and blindness.1 Two interventions that are commonly used to protect the eyes are instilling saline or methylcellulose lubricating drops and taping the eyelids in the shut position. Evidence suggests that an alternative technique may be more effective in preventing corneal epithelial breakdown. In addition to instilling saline drops every 2 hours, a polyethylene film is taped over the eyes, extending beyond the orbits and eyebrows. The film creates a moisture chamber around the cornea and assists in keeping the eyes moist and in the closed position. This technique also prevents damage to the eyes that results from tape or gauze being placed directly on the delicate skin of the eyelids.13 Collaborative management of the patient in coma is outlined in Box 24-4. Stroke is a descriptive term for the sudden onset of acute neurologic deficit persisting for more than 24 hours and caused by the interruption of blood flow to the brain. Stroke is the fourth leading cause of death in the United States, preceded by heart disease, cancer, and chronic respiratory disease. Each year, approximately 795,000 people have a stroke; 610,000 of these are first attacks, and 185,000 are recurrent attacks.14 Strokes are classified as ischemic and hemorrhagic (Fig. 24-1). Hemorrhagic strokes can be further categorized as subarachnoid hemorrhages (SAHs) and intracerebral hemorrhages (ICHs). Approximately 87% of all strokes are ischemic, 10% are ICHs, and 3% are SAHs. Although less common, hemorrhagic strokes (ICHs and SAHs) have a higher mortality rate compared with ischemic strokes. Approximately 8% to 12% of ischemic strokes and 37% to 38% of hemorrhagic strokes result in death within 30 days.14 The annual cost for care and loss of productivity was estimated to be $76.6 billion in 2012.14 Ischemic stroke results from interruption of blood flow to the brain and accounts for 80% to 85% of all strokes. The interruption can be the result of a thrombotic or embolic event. Thrombosis can form in large vessels (large-vessel thrombotic strokes) or small vessels (small-vessel thrombotic strokes). Embolic sources include the heart (cardioembolic strokes) and atherosclerotic plaques in larger vessels (atheroembolic strokes). In 30% of the cases, the underlying cause of the stroke is unknown (cryptogenic strokes).15 Strokes are preventable. Most thrombotic strokes are the result of the accumulation of atherosclerotic plaque in the vessel lumen, especially at the bifurcations or curves of the vessel. The pathogenesis of cerebrovascular disease is identical to that of coronary vasculature. The greatest risk factor for ischemic stroke is hypertension.15,16 Other risk factors are dyslipidemia, diabetes, smoking, and carotid atherosclerotic disease.14,17 Common sites of atherosclerotic plaque are the bifurcation of the common carotid artery, the origins of the middle and anterior cerebral arteries, and the origins of the vertebral arteries.16 Ischemic strokes resulting from vertebral artery dissection have been reported after chiropractic manipulation of the cervical spine.18 An embolic stroke occurs when an embolus from the heart or lower circulation travels distally and lodges in a small vessel, obstructing the blood supply. At least 20% of ischemic strokes are attributed to a cardioembolic phenomenon.15 The most common cause of cardiac emboli is atrial fibrillation. It is responsible for about 50% of all cardiac emboli.19 Other sources of cardiac emboli are from mitral stenosis, mechanical valves, atrial myxoma, endocarditis, and recent myocardial infarction.16 Researchers hypothesize that a patent foramen ovale or atrial septal aneurysms may be the cause of cryptogenic stroke.20 Ischemic stroke is a cerebral hemodynamic insult. When cerebral blood flow is reduced to a level insufficient to maintain neuronal viability, ischemic injury occurs. In focal stroke, an area of hypoperfused tissue, the ischemic penumbra, surrounds a core of ischemic cells. The ischemic penumbra can be salvaged with return of blood flow. However, sustained anoxic insult initiates a chain of biochemical events leading to apoptosis, or cellular death.21 The phenomenon of a focal ischemic stroke is identical to that associated with myocardial infarction, which is why the term brain attack is used in public education strategies. Often, a history of transient ischemic attacks (TIAs), brief episodes of neurologic symptoms that last less than 24 hours, offers a warning that stroke is likely to occur. Sudden onset indicates embolism as the final insult to flow.15,16 The size of the stroke depends on the size and location of the occluded vessel and the availability of collateral blood flow. Global ischemia results when severe hypotension or cardiopulmonary arrest provokes a transient drop in blood flow to all areas of the brain.21 Cerebral edema sufficient to produce clinical deterioration develops in 10% to 20% of patients with ischemic stroke and can result in intracranial hypertension. The edema results from a loss of normal metabolic function of the cells and peaks at 4 days.15 This process is commonly the cause of death during the first week after a stroke.22 Secondary hemorrhage at the site of the stroke lesion, known as hemorrhagic conversion, and seizures are the two other major acute neurologic complications of ischemic stroke.22,23 The characteristic sign of an ischemic stroke is the sudden onset of focal neurologic signs persisting for more than 24 hours.15 These signs usually occur in combination. Box 24-5 lists common patterns of neurologic symptoms associated with an ischemic stroke. Hemiparesis, aphasia, and hemianopia are common. Changes in the level of consciousness usually occur only with brainstem or cerebellar involvement, seizure, hypoxia, hemorrhage, or elevated intracranial pressure (ICP). These changes may be exhibited as stupor, coma, confusion, and agitation.1 The reported frequency of seizures in patients with ischemic stroke ranges from 3% to 8%. If seizures occur, they are usually seen within 24 hours of an insult.23 The National Institutes of Health Stroke Scale (NIHSS) is often used as the basis of the focused neurologic examination. The score ranges from 0 to 42 points; the higher the score, the more neurologically impaired the patient is. A change of 4 points on the scale indicates significant neurologic change. The components of the NIHSS include level of consciousness (LOC); LOC questions; LOC commands; gaze; visual fields; face, arm, and leg strength; sensation; limb ataxia; and language function.15 A copy of the NIHSS with complete instructions is available at http://www.ninds.nih.gov/disorders/stroke/strokescales.htm. Confirmation of the diagnosis of ischemic stroke is the first step in the emergent evaluation of these patients. Differentiation from intracranial hemorrhage is vital. Noncontrast computed tomography (CT) scanning is the method of choice for this purpose, and it is considered the most important initial diagnostic study. In addition to excluding intracranial hemorrhage, CT can assist in identifying early neurologic complications and the cause of the insult.15 Magnetic resonance imaging (MRI) can demonstrate infarction of cerebral tissue earlier than can CT but is less useful in the emergent differential diagnosis.24 Because of the strong correlation between acute ischemic stroke and heart disease, 12-lead electrocardiography (ECG), chest radiography, and continuous cardiac monitoring are suggested to detect a cardiac cause or coexisting condition. Echocardiography is valuable in identifying a cardioembolic phenomenon when a sufficient index of suspicion warrants its use.1 Laboratory evaluation of hematologic function, electrolyte and glucose levels, and renal and hepatic function is also recommended. Arterial blood gas analysis is performed if hypoxia is suspected, and electroencephalography (EEG) is performed if seizures are suspected. Lumbar puncture is performed only if SAH is suspected and the CT scan is normal.1 Major changes have taken place in the medical management of ischemic stroke since 1996. Based on results of the National Institute of Neurologic Disorders and Stroke (NINDS) recombinant tissue plasminogen activator (rtPA) Stroke Study, fibrinolytic therapy with intravenous rtPA is recommended within 3 hours of onset of ischemic stroke.20 This time frame has now been expanded from 3 hours to 4.5 hours.25 Patients who should be considered for fibrinolysis are listed in Box 24-6. Confirmation of diagnosis with CT must be accomplished before rtPA administration. The recommended dose of rtPA is 0.9 milligram per kilogram (mg/kg) up to a maximum dose of 90 mg. Ten percent of the total dose is administered as an initial intravenous bolus, and the remaining 90% is administered by intravenous infusion over 60 minutes.20,24 The desired result of fibrinolytic therapy is to dissolve the clot and reperfuse the ischemic brain. The goal is to reverse or minimize the effects of stroke. The major risk and complication of rtPA therapy is bleeding, especially intracranial hemorrhage. Unlike fibrinolytic protocols for acute myocardial infarction, subsequent therapy with anticoagulant or antiplatelet agents is not recommended after rtPA administration in ischemic stroke. Patients receiving fibrinolytic therapy for stroke should not receive aspirin, heparin, warfarin, ticlopidine, or any other antithrombotic or antiplatelet medications for at least 24 hours after treatment.15,20 The major barriers to effective application of fibrinolytic therapy for ischemic stroke are prehospital and in-hospital delays.26 To help decrease delays, the public needs to be educated about stroke symptoms and activation of emergency medical system (EMS). EMS responders need adequate education and training on managing a patient with an acute ischemic stroke, focusing on stabilization and transport of the patient quickly to the emergency department. The receiving hospital should ideally have certification for primary stroke treatment and have expert staff and the infrastructure to care for the patient with complex stroke.20,24 Other emergent care of the patient with ischemic stroke must include airway protection and ventilatory assistance to maintain adequate tissue oxygenation.22 Hypertension is often present in the early period as a compensatory response, and in most cases, blood pressure (BP) must not be lowered (Table 24-1). For the patient who has not received fibrinolytic therapy, antihypertensive therapy is considered only if the diastolic blood pressure is greater than 120 mm Hg or the systolic blood pressure is greater than 220 mm Hg.15,20 Criteria are different for patients who have received rtPA. Their blood pressure is kept below 180/105 mm Hg to prevent intracranial hemorrhage. Intravenous labetalol or nicardipine is used to achieve blood pressure control. If these agents are not effective, nitroprusside, hydralazine, or enalaprilat should be considered.15 Body temperature and glucose levels also must be normalized.15,22 TABLE 24-1 BLOOD PRESSURE MANAGEMENT FOR STROKE ACCORDING TO THE AMERICAN STROKE ASSOCIATION GUIDELINES *All initial blood pressures should be verified before treatment by repeating reading in 5 minutes. †As estimated by one third of the sum of systolic and double diastolic pressure. ‡Labetalol should be avoided in patients with asthma, cardiac failure, or severe abnormalities in cardiac conduction. For refractory hypertension, alternative therapy may be considered with sodium nitroprusside or enalapril. From Bader MK, Littlejohns LR. AANN Core Curriculum for Neuroscience Nursing. 4th ed. St. Louis: Elsevier; 2004. Medical management also includes the identification and treatment of acute complications such as cerebral edema or seizure activity. Prophylaxis for these complications is not recommended. Deep vein thrombosis (DVT) prophylaxis, however, should be initiated to decrease the risk of pulmonary embolism.15 One study demonstrated that improved outcomes for patients with ischemic stroke can be achieved by managing swallowing issues, initiating DVT prophylaxis, and treating hypoxemia.27 Surgical decompression is recommended if a large cerebellar infarction compresses the brainstem.22 Subarachnoid hemorrhage (SAH) is bleeding into the subarachnoid space, which usually is caused by rupture of a cerebral aneurysm or arteriovenous malformation (AVM).22 At the time of autopsy, approximately 4% of the population has been found to have one or more aneurysms.28 With improvements in imaging techniques, an increased number of incidental intracranial aneurysms has been found. Computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) can detect up to 95% of all aneurysms. Among people younger than 40 years, more men than women are likely to have SAHs, whereas among those older than 40 years, more women have SAHs. Aneurysmal SAH is associated with a mortality rate of 25% to 50%, with most patients dying on the first day after the insult.28 Hemorrhage from AVM rupture has a better chance of survival and is associated with an overall mortality rate of 10% to 15%.29 Known risk factors for SAH include hypertension, smoking, and alcohol or stimulant use.30 Cerebral aneurysm rupture accounts for approximately 85% of all cases of spontaneous SAH.28 An aneurysm is an outpouching of the wall of a blood vessel that results from weakening of the wall of the vessel (Table 24-2).28 Ninety percent of aneurysms are congenital—the cause of which is unknown. The other 10% can be the result of traumatic injury (that stretches and tears the muscular middle layer of the arterial vessel) or infectious material (most often from infectious vegetation on valves of the left side of the heart after bacterial endocarditis) that lodges against a vessel wall and erodes the muscular layer, or they are of undetermined cause.30 Multiple aneurysms occur in approximately 30% of the cases and often are bilateral, occurring in the same location on both sides of the cerebral vascular system.31 TABLE 24-2 ANEURYSM CLASSIFICATION ACCORDING TO TYPE, SHAPE, LOCATION, AND COMMON CHARACTERISTICS AVM rupture is responsible for roughly 6% of all SAHs.31 An AVM is a tangled mass of arterial and venous blood vessels that shunt blood directly from the arterial side into the venous side, bypassing the capillary system. AVMs may be small, focal lesions or large, diffuse lesions that occupy almost an entire hemisphere.30 They are always congenital, although the exact embryonic cause for these malformations is unknown. They also occur in the spinal cord and the renal, gastrointestinal, and integumentary systems.31 Small, superficial AVMs are seen as port-wine stains of the skin. In contrast to the SAH from an aneurysm in the middle-aged population, SAH from an AVM usually occurs in the second to fourth decades of life.30,31 As the individual with a congenital cerebral aneurysm gets older, blood pressure rises, and more stress is placed on the poorly developed and thin vessel wall. Ballooning of the vessel occurs, giving the aneurysm a berrylike appearance. Most cerebral aneurysms are saccular or berrylike, with a stem or neck. Aneurysms are usually small, are 2 to 7 millimeters (mm) in diameter, and often occur at the base of the brain on the circle of Willis.29 Figure 24-2 illustrates the usual distribution between the vessels. Most cerebral aneurysms occur at the bifurcation of blood vessels.28,29,32 The aneurysm becomes clinically significant when the vessel wall becomes so thin that it ruptures, sending arterial blood at a high pressure into the subarachnoid space. For a brief moment after the aneurysm ruptures, ICP is thought to approach mean arterial pressure, and cerebral perfusion decreases.32 In other situations, the unruptured aneurysm expands and places pressure on surrounding structures. This is particularly true with posterior communicating artery aneurysms because they put pressure on the oculomotor nerve (cranial nerve III), causing ipsilateral pupil dilation and ptosis.31 The pathophysiologic features of an AVM are related to the size and location of the malformation. One or more cerebral arteries, also known as feeders, supply an AVM. These feeder arteries tend to enlarge over time and increase the volume of blood shunted through the malformation and increase the overall mass effect. Large, dilated, tortuous draining veins develop as a result of increasing arterial blood flow being delivered at a higher-than-normal pressure. Normal vascular flow has a mean arterial pressure of 70 to 80 mm Hg, a mean arteriole pressure of 35 to 45 mm Hg, and a mean capillary pressure that drops from 35 to 10 mm Hg as it connects with the venous side. Lack of this capillary bridge allows blood with a mean pressure of 35 to 45 mm Hg to flow into the venous system. Unlike arteries, veins have no muscular layer and become extremely engorged and rupture easily. Some patients with AVMs also have cerebral atrophy. It is the result of chronic ischemia because of the shunting of blood through the AVM and away from normal cerebral circulation.33 The patient with an SAH characteristically has an abrupt onset of pain, described as the “worst headache of my life.” A brief loss of consciousness, nausea, vomiting, focal neurologic deficits, and a stiff neck may accompany the headache.28–32 The SAH may result in coma or death. The patient’s history may reveal one or more incidences of sudden onset of headache with vomiting in the weeks preceding a major SAH. These are small “warning leaks” of an aneurysm in which small amounts of blood ooze from the aneurysm into the subarachnoid space. The presence of blood is an irritant to the meninges, particularly the arachnoid membrane, and the irritation causes headache, stiff neck, and photophobia. These warning leaks seldom are detected because the condition is not severe enough for the patient to seek medical attention.32 If a neurologic deficit such as third cranial nerve palsy develops before aneurysm rupture, medical intervention is sought, and the aneurysm may be surgically secured before the devastation of a rupture can occur. Symptoms of unruptured AVM—headaches with dizziness or syncope or fleeting neurologic deficits—also may be found in the history.31 Diagnosis of SAH is based on clinical presentation, CT findings, and lumbar puncture results. Noncontrast CT is the cornerstone of definitive SAH diagnosis.30–34 In 95% of the cases, CT demonstrates blood in the subarachnoid space if performed within 48 hours the hemorrhage.28,32 On the basis of the appearance and the location of the SAH, diagnosis of the cause—aneurysm or AVM—may be made from the CT scan. MRI is not routinely used, but it may provide greater sensitivity for detecting the areas of SAH clot and the potential location of the bleed.32 If the initial CT finding is negative, a lumbar puncture is performed to obtain cerebrospinal fluid (CSF) for analysis. CSF after SAH appears bloody and has a red blood cell count greater than 1000 cells/mm3. If the lumbar puncture is performed more than 5 days after the SAH, the CSF fluid is xanthochromic (dark amber) because the blood products have broken down.33 Cloudy CSF usually indicates some type of infectious process such as bacterial meningitis, not SAH.31 After the SAH has been documented, cerebral angiography is necessary to identify the exact cause of the hemorrhage (Fig. 24-3). If a cerebral aneurysm rupture is the cause, angiography is essential for identifying the exact location of the aneurysm in preparation for surgery.31,33,34 After the aneurysm has been located, it is graded using the Hunt and Hess classification scale. This scale categorizes the patient on the basis of the severity of the neurologic deficits associated with the hemorrhage (Box 24-7).35 If AVM rupture is the cause, angiography is necessary to identify the feeding arteries and draining veins of the malformation.30 SAH is a medical emergency, and time is of the essence. Preservation of neurologic function is the goal, and early diagnosis is crucial. Initial treatment must always support vital functions. Airway management and ventilatory assistance may be necessary.22 A ventriculostomy is performed to control ICP if the patient’s level of consciousness is depressed.32,34 Evidence suggests that only 19% of the deaths attributable to aneurysmal SAH are related to the direct effects of the initial hemorrhage.36 Rebleeding accounts for 22% of deaths from aneurysmal SAH, cerebral vasospasm for 23%, and non-neurologic medical complications for 23%.36 Principal non-neurologic causes of death are systemic inflammatory response syndrome (SIRS) and secondary organ dysfunction.37 After initial intervention has provided the necessary support for vital physiologic functions, medical management of acute SAH is aimed primarily toward the prevention and treatment of the complications of SAH, which may produce further neurologic damage and death.32 Rebleeding is the occurrence of a second SAH in an unsecured aneurysm or, less commonly, an AVM.28 The incidence of rebleeding during the first 24 hours after the first bleed is 4%, with a 1% to 2% chance per day in the following month. The mortality rate associated with aneurysmal rebleeding is approximately 70%.30,31 Historically, conservative measures to prevent rebleeding have included blood pressure control and SAH precautions (see “Nursing Management”). An elevation in blood pressure is a normal compensatory response to maintain adequate cerebral perfusion after a neurologic insult. In the belief that hypertension contributes to rebleeding, nitroprusside, metoprolol, or hydralazine has been commonly used to maintain a systolic blood pressure no greater than 140 mm Hg.32 Individualized guidelines must be determined on the basis of the clinical condition and pre-existing values of the patient. Evidence suggests that rebleeding has more to do with variations in blood pressure than it does with absolute values and that blood pressure control does not lower the incidence of rebleeding.34 Definitive treatment for the prevention of rebleeding is surgical clipping or endovascular coiling with complete obliteration of the aneurysm.31,32 Timing of the operation is a key medical management issue. Since the introduction of microsurgery and improved surgical techniques, patients commonly are taken to the operating room within the first 48 hours after rupture.32 This early surgical intervention to secure the aneurysm eliminates the risk of rebleeding and allows more aggressive therapy to be used in the postoperative period for the treatment of vasospasm.31,32 Early surgery also allows the neurosurgeon to flush out the excess blood and clots from the basal cisterns (reservoir of CSF around the base of the brain and circle of Willis) to reduce the risk of vasospasm.37 Careful consideration of the patient’s clinical situation is necessary in determining the optimal time for surgery. The surgical procedure involves a craniotomy to expose and isolate the area of aneurysm. A clip is placed over the neck of the aneurysm to eliminate the area of weakness (Fig. 24-4). This is a technically difficult procedure that requires the skill of an experienced neurosurgeon. It is not uncommon, particularly in early surgery, for the clot to break away from the aneurysm as it is surgically exposed. Extensive hemorrhage into the craniotomy site results, and cessation of the hemorrhage often causes increased neurologic deficits. Deficits also may occur as a result of surgical manipulation to gain access to the site of the aneurysm.32 Management of AVM has traditionally involved surgical excision or conservative management of such symptoms as seizures and headache. The decision for surgical excision depends on the location and size of the AVM. Some malformations are located so deep in the cerebral structures (thalamus or midbrain) that attempts to remove the AVM would cause severe neurologic deficits. History of a previous hemorrhage and the patient’s age and overall condition are also taken into account when making the decision regarding surgical intervention.32 Surgical excision of large AVMs includes the risk of reperfusion bleeding. As feeding arteries of the AVM are clamped off, the arterial blood that usually flowed into the AVM is diverted into the surrounding circulation. In many cases, the surrounding tissue has been in a state of chronic ischemia, and the arterial vessels feeding these areas are maximally dilated. As arterial blood begins to flow at a higher volume and pressure into these dilated arteries, blood may seep from the vessels. Evidence of reperfusion bleeding in the operating room is an indication that no more arterial blood can be diverted from the AVM without risk of serious ICH. In the postoperative phase, a low blood pressure is maintained to prevent further reperfusion bleeding. For large AVMs, two to four stages of surgery may be required over 6 to 12 months.32 Embolization is used to secure a cerebral aneurysm or AVM that is surgically inaccessible because of size, location, or medical instability of the patient. Embolization involves several new interventional neuroradiology techniques. All of the techniques use a percutaneous transfemoral approach in a manner similar to an angiography. Under fluoroscopy, the catheter is threaded up to the internal carotid artery. Specially developed microcatheters are then manipulated into the area of the vascular anomaly, and embolic materials are placed endovascularly. Three embolization techniques are used, depending on the underlying pathologic derangement.30 The first type of embolization is used to embolize an AVM. Small polymeric silicone (Silastic) beads or glue is slowly introduced into the vessels feeding the AVM. Blood flow carries the material to the site, and embolization is achieved. This procedure may be used in combination with surgery. One to three sessions of embolization of the feeding vessels are performed to reduce the size of the lesion before a craniotomy is performed for total excision. The primary risk of this procedure is lodging of the embolic substance in a vessel that feeds normal tissue, which creates an embolic stroke with the immediate onset of neurologic symptoms.30 The second type of embolization involves placement of one or more detachable coils into an aneurysm to produce an endovascular thrombus (Fig. 24-5). The advantage of this technique is that an electrical current creates a positive charge on the coil, which induces electrothrombosis. Complications include embolic stroke, coil migration, overproduction of the clot, subtotal occlusion and intraprocedural rupture of the vasculature, and death.30 The presence or absence of cerebral vasospasm significantly affects the outcome of aneurysmal SAH. This complication does not occur with SAH resulting from AVM rupture. Cerebral vasospasm is a narrowing of the lumen of the cerebral arteries, possibly in response to subarachnoid blood clots coating the outer surface of the blood vessels. Because aneurysms usually occur at the circle of Willis, the major vessels responsible for feeding the cerebral circulation are affected by vasospasm. Depending on the arterial vessels involved in the vasospasm reaction, decreased arterial flow occurs in large areas of the cerebral hemispheres.30 It is estimated that 70% of all SAH patients develop vasospasm, which is demonstrable by angiography.38 Thirty-two percent of these patients develop symptomatic vasospasm, resulting in ischemic stroke or death for up to 23% of them despite the use of maximal therapy.38 The onset of vasospasm is usually 3 to 12 days after the initial hemorrhage.22 Three treatments are commonly used: 1) induced hypertensive, hypervolemic, hemodilution (HHH) therapy; 2) oral nimodipine; and 3) transluminal cerebral angioplasty.34,38 HHH therapy involves increasing the patient’s blood pressure and cardiac output with vasoactive medications and diluting the patient’s blood with fluid and volume expanders. Systolic blood pressure is maintained between 150 and 160 mm Hg. The increase in volume and pressure forces blood through the vasospastic area at higher pressures. Hemodilution facilitates flow through the area by reducing blood viscosity. Many anecdotal reports exist of patients’ neurologic deficits improving as systolic pressure increases from 130 mm Hg to between 150 and 160 mm Hg.39 The Stroke Council of the AHA has recommended this therapy for prevention and treatment of vasospasm.34 The obvious deterrent to the use of induced hypertension is the risk of rebleeding in an unsecured aneurysm. Surgical clipping of the aneurysm before HHH therapy is preferred. Cerebral edema, elevated ICP, heart failure, and electrolyte imbalance are also risks of HHH therapy. Careful monitoring of the patient’s neurologic status, hemodynamic parameters, ICP, and serum electrolytes is necessary.32 Nimodipine is strongly recommended to reduce the poor outcomes associated with vasospasm. The exact nature of the effect of nimodipine is not clear, but the use of the medication has demonstrated consistently positive effects on outcome without any demonstrable effect on the incidence or severity of vasospasm.34,38 A dose of 60 mg of nimodipine is given orally every 4 hours for 21 days. Nimodipine may produce hypotension, especially when administered concurrently with other antihypertensive agents.38 Cerebral angioplasty is used when pharmacologic management of cerebral vasospasm has failed. It is performed only when CT or MRI provides evidence that infarction has not occurred. An interventional neuroradiologist performs the procedure, and the patient is placed under local, general, or neuroleptic analgesia. The technique of cerebral angioplasty is very similar to that used in the coronary vasculature. Risks include intimal perforation or rupture, cerebral artery thrombosis or embolism, recurrence of stenosis, and severe, diffuse vasospasm unresponsive to therapy. Hemorrhage at the femoral site also may occur. This procedure is recommended when conventional therapy is unsuccessful.34,38 Hyponatremia develops in 10% to 43% of patients with SAH as the result of central salt-wasting syndrome. It usually occurs during the same period as vasospasm, several days after the initial hemorrhage.29 The use of fluid restriction to treat hyponatremia in the SAH patient is associated with a poor outcome. The AHA Stroke Council strongly recommends that fluid restriction not be used in this instance and instead recommends sodium replenishment with isotonic fluids.34 Hydrocephalus is a late complication that occurs in approximately 25% of patients after SAH.30 Blood that has circulated in the subarachnoid space and has been absorbed by the arachnoid villi may obstruct the villi and reduce the rate of CSF absorption. Over time, increasing volumes of CSF in the intracranial space produce communicating hydrocephalus. Treatment consists of placing a drain to remove CSF. This can be accomplished temporarily, by inserting a ventriculostomy, or permanently, by placing a ventriculoperitoneal shunt.26,31,34 Intracerebral hemorrhage (ICH) is bleeding directly into cerebral tissue.40 ICH destroys cerebral tissue, causes cerebral edema, and increases ICP. The source of intracerebral bleeding is usually a small artery, but it can result also from rupture of an AVM or aneurysm. The most important cause of spontaneous ICH is hypertension, and this section concentrates on spontaneous hypertensive ICH.41 Spontaneous ICH accounts for at least 10% of all stroke admissions.40 The likelihood of death or disability is higher with ICH than with ischemic stroke or SAH. The mortality rate for hemorrhagic stroke is up to 50% within 1 month. Only 20% of patients with ICH return to a functional life at 6 months.42 The key risk factors for ICH are age-associated cerebral amyloid angiopathy and hypertension.40 ICH is most often caused by hypertensive rupture of a cerebral vessel, resulting from a longstanding history of hypertension.40 Other possible causes of spontaneous ICH are anticoagulation or fibrinolytic therapy, coagulation disorders, drug abuse, and hemorrhage into a cerebral infarct or brain tumors.31,43 Often, on questioning, the patient with a hypertensive hemorrhage admits to having discontinued antihypertensive medication 2 to 3 weeks before the hemorrhage. The pathophysiology of ICH is caused by continued elevated blood pressure exerting force against smaller arterial vessels that have become damaged from arteriosclerotic changes. Eventually, these arteries break, and blood bursts from the vessels into the surrounding cerebral tissue, creating a hematoma. ICP rises precipitously in response to the increase in overall intracranial volume.43 Initial assessment usually reveals a critically ill patient who often is unconscious and requires ventilatory support. History from a relative or significant other describes a sudden onset of focal deficit often accompanied by severe headache, nausea, vomiting, and rapid neurologic deterioration. Signs and symptoms vary, depending on the location of the ICH.43 Approximately 50% of patients sustain early loss of consciousness, a key feature that differentiates ICH from ischemic stroke. More than one half of the patients with ICH present with a smooth progression of neurologic symptoms, an uncommon finding in cases of ischemic stroke or SAH.44 One third of the patients have maximal symptoms at onset. Assessment of vital signs usually reveals a severely elevated blood pressure (200/100 to 250/150 mm Hg). Signs of increased ICP are often present by the time the patient arrives in the emergency department. Diagnosis is established easily with CT. Angiography is recommended only in patients considered surgical candidates and if a clear cause of hemorrhage is not evident.40–44 ICH is a medical emergency. Initial management requires attention to airway, breathing, and circulation. Intubation is usually necessary. Blood pressure management must be based on individual factors. Reduction in blood pressure is usually necessary to decrease ongoing bleeding, but lowering blood pressure too much or too rapidly may compromise cerebral perfusion pressure (CPP), especially in the patient with elevated ICP. National guidelines recommend keeping the mean arterial blood pressure below 130 mm Hg in patients with a history of hypertension by moderate blood pressure reduction to a mean arterial pressure below 110 mm Hg.44 Vasopressor therapy after fluid replenishment is recommended if systolic blood pressure falls below 90 mm Hg.41 Increased ICP is common with ICH and is a major contributor to mortality. Recommended management includes mannitol, when indicated; hyperventilation; and neuromuscular blockade with sedation. Steroids are avoided. CPP must be kept higher than 70 mm Hg.41,42 The goal for fluid management is euvolemia, with a recommended pulmonary artery occlusion pressure (PAOP) of 10 to 14 mm Hg. Body temperature is maintained at less than 38.5° C by using acetaminophen or cooling blankets. Euglycemia, a blood glucose level less than 140 milligram per deciliter (mg/dL), is maintained by using insulin therapy, but hypoglycemia should be avoided. Use of short-acting benzodiazepines or propofol is recommended to treat agitation or hyperactivity. Pneumatic compression devices are used to decrease risk of pulmonary embolism. Prophylactic anticonvulsant therapy is sometimes used.41,44 The benefit of surgical treatment for spontaneous ICH is unclear. Recommendations for surgical removal of the clot depend on the size and location of the hematoma, the patient’s ICP, and other neurologic symptoms.44 Medical treatment is recommended if the hemorrhage is small (<10 cm) or neurologic deficit is minimal.41,44 Likewise, surgery offers no improvement in outcome for patients with a Glasgow Coma Scale (GCS) score of 4 or less. Surgical evacuation of the clot is recommended for patients with cerebellar hemorrhage greater than 3 cm with neurologic deterioration or hydrocephalus with brainstem compression, as well as for young patients with moderate or large lobar hemorrhage with clinical deterioration.41,44 Numerous techniques are being investigated to lessen the risk of brain damage associated with craniotomy for ICH. Evidence-based guidelines for the management of the patient with ICH are listed in Box 24-8.
Neurologic Disorders and Therapeutic Management
Coma
Description
Etiology
Pathophysiology
Assessment and Diagnosis
Medical Management
Nursing Management
Eye Care
Stroke
Ischemic Stroke
Description
Etiology
Pathophysiology
Assessment and Diagnosis
Medical Management
BLOOD PRESSURE*
TREATMENT
Nonthrombolytic Candidates
DBP >140 mm Hg
Sodium nitroprusside (0.5 mcg/kg/min); aim for 10%-20% reduction in DBP
SBP >220 mm Hg, DBP 121-140 mm Hg, or MAP† >130 mm Hg
10-20 mg of labetalol‡ given by IVP over 1-2 min; may repeat or double labetalol every 20 min to a maximum dose of 300 mg
SBP <220 mm Hg, DBP = 120 mm Hg, or MAP† <130 mm Hg
Emergency antihypertensive therapy is deferred in the absence of aortic dissection, acute myocardial infarction, severe congestive heart failure, or hypertensive encephalopathy
Thrombolytic Candidates
Pretreatment
SBP >185 mm Hg or DBP >110 mm Hg
1-2 inches of nitroglycerine paste (Nitropaste) or 1-2 doses of 10-20 mg of labetalol‡ given by IVP; if BP is not reduced and maintained to <185/110 mm Hg, the patient should not be treated with tPA
During and After Treatment
Monitor BP
BP is monitored every 15 min for 2 hr, then every 30 min for 6 hr, and then hourly for 16 hr
DBP >140 mm Hg
Sodium nitroprusside (0.5 mcg/kg/min)
SBP >230 mm Hg or DBP 121-140 mm Hg
10 mg of labetalol‡ given by IVP over 1-2 min; may repeat or double labetalol every 10 min to a maximum dose of 300 mg or give initial labetalol bolus and then start a labetalol drip at 2-8 mg/min
If BP not controlled by labetalol, consider sodium nitroprusside
SBP 180-230 mm Hg or DBP 105-120 mm Hg
10 mg of labetalol‡ given by IVP; may repeat or double labetalol every 10-20 min to a maximum dose of 300 mg or give initial labetalol bolus and then start a labetalol drip at 2-8 mg/min
Subarachnoid Hemorrhage
Description
Etiology
TYPES OF ANEURYSMS
CHARACTERISTICS
Berry or saccular
Most common type, usually congenital; appears at a bifurcation in the anterior circulation, primarily at the base of the brain or the circle of Willis and its branches; grows from the base of the arterial wall with a neck or stem; contains blood; thinned dome is usually the site of rupture
Giant or fusiform

Can have an irregular shape and be larger than 2.5 cm and atherosclerotic; involves mainly the internal carotid or vertebrobasilar artery; rarely ruptures; has no stem; may act like a space-occupying lesion in the brain; difficult to manage
Mycotic

Rare form; usually occurs from septic emboli, usually results from bacterial infection, which weaken the vessel wall, causing dilation involving the distal branches of the middle cerebral arteries
Dissecting
May occur during angiography; caused by trauma, syphilis, arteriosclerosis, or when blood is forced between layers of the arterial wall; intima is pulled away from the medial layer, allowing blood to enter
Traumatic
Sometimes called a pseudoaneurysm, which may resolve after trauma
Charcot-Bouchard
Small aneurysm that can be seen in the area of the basal ganglia or the brainstem in individuals with a history of hypertension; chronic hypertension causes fibrinoid necrosis in the penetrating and subcortical arteries, weakening the arterial walls and causing formation of small aneurysmal outpouching
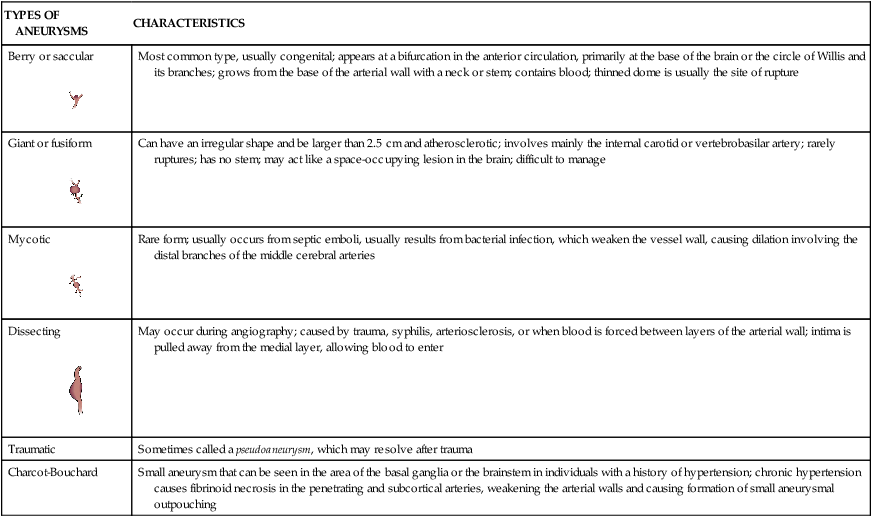
Pathophysiology
Cerebral Aneurysm.
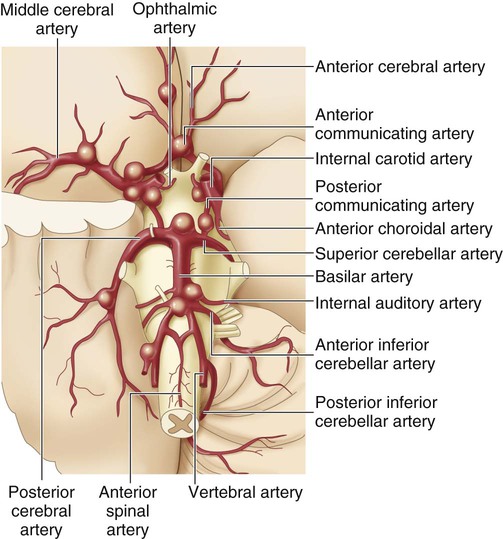
The size of the aneurysm in the drawing is proportional to the frequency of occurrence at the various sites. (From Zivin JA. Hemorrhagic cerebrovascular disease. In: Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine. 24th ed. St. Louis: Elsevier; 2012.)
Arteriovenous Malformation.
Assessment and Diagnosis
Medical Management
Rebleeding.
Surgical Clipping of Aneurysms.
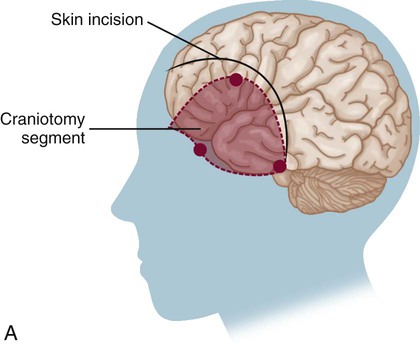
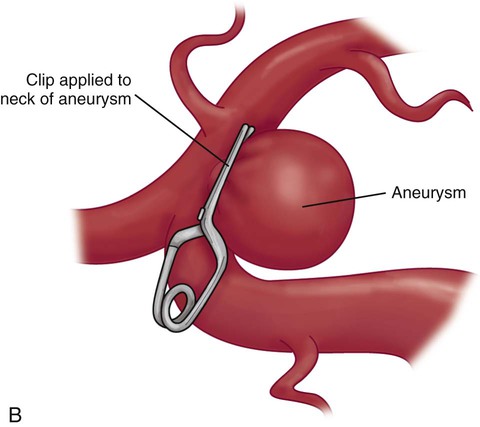
A, The solid curved line shows the typical skin incision, and the dashed lines show the craniotomy location. B, Application of the clip to the aneurysm.
Surgical Excision of Arteriovenous Malformations.
Embolization.
Cerebral Vasospasm.
Hypertensive, Hypervolemic, Hemodilution Therapy.
Nimodipine.
Cerebral Angioplasty.
Hyponatremia.
Hydrocephalus.
Intracerebral Hemorrhage
Description
Etiology
Pathophysiology
Assessment and Diagnosis
Medical Management