anatomic imaging reveals information about the structure of the nervous system, including the brain and spinal cord. Functional or physiologic imaging focuses on the function of the brain and biochemical and metabolic processes in brain cells.
Use age-appropriate assessment and examination techniques.
Be aware of the status of the patient when assuming care so comparison can be made with subsequent assessments.
Perform a thorough systematic assessment, including mental status, vital signs, and cardiovascular status.
Document the patient’s condition to provide a record for continuity of care.
Provide translation services for patients who have difficulty understanding or speaking English because this may impact the interpretation of the neurologic exam.
Evaluate for signs of worsening neurologic condition through a systematic examination. Follow the institutional guidelines and clinician’s orders regarding the frequency of assessments. If the patient shows signs of neurologic deterioration, more frequent assessment and/or interventions may be necessary.
Be cautious in the administration of sedatives, opiates, or other medications that affect neurologic functioning because these may mask signs of neurologic deterioration.
Notify appropriate health care provider of new or worsening neurologic symptoms, such as a change in behavior or level of consciousness; a change in functioning of the cranial nerves; motor, sensory, or neurovascular deficits; or alterations in the pattern of breathing or vital signs. Implement appropriate interventions for acute changes in neurologic status.
Institute safety precautions for patients with neurologic deficits because they may be especially prone to falls. Follow institutional guidelines for patients with seizure disorder.
Assess the patient’s level of functioning in his or her activities of daily living. For those with cranial nerve or motor deficits, assess for difficulty swallowing prior to eating. For patients with difficulty swallowing, implement appropriate institutional protocols to prevent aspiration. This may include contacting the primary clinician to obtain an evaluation from a speech therapist or changing the diet.
Use a multidisciplinary approach to care when indicated, including medical and surgical specialists, pharmacists, dietitian/nutritional therapist, physical/occupational/speech therapists, and rehabilitation specialists.
Be aware that families or caretakers of patients with cognitive impairment or who are nonverbal may be able to provide assistance in the interpretation of behavioral cues.
Assess family support and coping throughout the trajectory of the disease. Social issues (such as financial, community support systems, etc.) may require the expertise of a social worker or other clinical resource support personnel.
 Evidence Base
Evidence Base
Computed tomography (CT) is a structural imaging study that uses a computer-based x-ray to provide a cross-sectional image of the brain. A computer calculates differences in tissue absorption of the x-ray beams. The CT scan produces a threedimensional view of structures in the brain and distinguishes between soft tissues and water. Intravenous (IV) contrast dye may be used to examine the integrity of the blood-brain barrier. New multidetector scanners allow for rapid imaging.
CT of the brain is primarily used to detect cerebral hemorrhage, whereas CT of the brain with contrast is used to detect tumors and inflammatory disorders.
Spinal CT may be used to evaluate lower back pain due to bony lesions or degenerative changes. CT myelography is typically reserved for those who have had previous spinal surgery or a questionable diagnosis.
May be used when magnetic resonance imaging (MRI) is contraindicated (metallic or electronic implants) or not tolerated (claustrophobia).
Advantages of CT: Widespread availability, short imaging time, excellent images of bone, and 100% sensitivity for detection of cerebral hemorrhage.
Disadvantages of CT: Does not provide information about function of tissues; exposes the patient to ionizing radiation at higher doses than traditional x-rays; imposes a weight restriction of 300 pounds; if contrast is used there is the risk of contrast-induced nephropathy.
Instruct patient to remove metal items, such as earrings, eyeglasses, and hair clips.
Ask whether patient has an allergy to iodine or history of previous allergy to IV dye to determine if the patient needs to be premedicated.
Evaluate for adequate renal function if contrast will be used.
Tell patient to expect a sensation of feeling flushed if contrast dye is injected through the IV catheter.
Inform patient that the procedure usually takes less than 5 minutes.
Request that patient remain as immobile as possible during the examination.
Tell patient to resume usual activities after the procedure.
Encourage increased fluid intake for the rest of the day to assist in expelling the contrast dye.
Conventional MRI is a noninvasive structural imaging procedure that uses powerful magnetic field and radio frequency waves to create an image. When tissue is placed in a strong magnetic field, hydrogen atoms in the tissue line up within the field. In MRI, pulsating radio frequency waves are applied to the magnetic field to alter the tissue magnetization, creating a clear image of the tissue.
MRI is the imaging procedure of choice for most neurologic diseases (eg, detection of demyelinating diseases, nonacute hemorrhage, and cerebral tumors; evaluation of spinal cord injury, acute herniated disks, and cerebral infarction) and has largely replaced myelography, a more invasive procedure. See Table 15-1 for comparison to CT.
Specific protocols have been developed for trauma, stroke, and epilepsy.
MRI may be ordered with or without contrast. The contrast (gadolinium) alters the magnetic properties of tissue in order to differentiate tissue types. Contrast may be administered via IV line, by mouth, or through insertion into the rectum.
Closed MRI uses scanning equipment that resembles a tunnel-like chamber. Open MRI uses more sophisticated equipment that does not involve a closed chamber. During open MRI, the patient can comfortably see the surroundings from all views while the scan is in progress. This is ideal for patients who are claustrophobic or anxious, children, older adults, and the very obese.
Imaging orientation:
Axial—slice dividing the head into upper and lower halves.
Coronal—slice dividing the head into front and back halves.
Sagittal—slice dividing the head into left and right halves.
Imaging sequences:
T1—excellent tissue discrimination that provides anatomic images.
T2—sensitive to the presence of increased water and visualization of edema for differentiation of normal tissue and pathologic changes.
MRI with gadolinium—improves specificity of normal/abnormal tissue. With blood-brain barrier disruption, there is leakage of contrast medium. The pattern of contrast uptake into brain tissue helps differentiate such conditions as central nervous system (CNS) infections, neoplasms, meningeal diseases, and noninfectious inflammatory processes.
Fluid attenuated inversion recovery—evaluates edema within white matter; also used to identify subarachnoid disease.
Diffusion-weighted imaging (DWI)—helps to evaluate the extent of a stroke and demyelinating disease and differentiates tumors from abscesses; shows increased enhancement with cytotoxic edema.
Apparent diffusion coefficient (ADC)—shows decreased attenuation associated with acute stroke.
DWI/ADC mismatch—increased enhancement on DWI in conjunction with decreased enhancement on ADC indicates acute stroke.
Perfusion-weighted imaging (PWI)—evaluates cerebral blood flow. It is also used to evaluate ischemia, stroke, and the penumbra of the stroke.
DWI/PWI mismatch—increased enhancement on DWI with flow deficit on PWI indicates acute stroke.
MRI single photon-emission computed tomography (SPECT)—provides assessment of neurochemicals (choline, lactate, N-acetylaspartate, glutamine/glutamate) for differentiation of brain tumors, abscess, demyelinating disease, and postradiation necrosis.
Advantages of MRI: No ionizing radiation, sensitivity to blood flow, imaging in several planes, and superior visualization of soft tissues. An important advantage is its ability to distinguish water, iron, fat, and blood. Sensitive to detection of white matter changes and valuable in detecting changes associated with Alzheimer’s disease and multiple sclerosis.
Disadvantages of MRI: Contraindicated for patients with pacemakers, nontitanium aneurysm clips, or other implanted objects that could be dislodged by the magnetic field. Dental amalgam, gold, and stainless steel are generally considered safe, but may distort the image. If contrast is used, there is the risk of contrast-induced nephropathy. MRI scanner has the appearance of a tunnel-like chamber, and its constricted opening prevents its use for extremely obese people. Because of its narrow dimensions, MRI may induce claustrophobic and anxiety reactions, so anti-anxiety medication may be necessary before the procedure or an open MRI may be used.
Table 15-1 Comparison of CT and MRI | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Encourage patient to use the bathroom before the procedure because it may take from 20 to 60 minutes, though the scan time is dependent on the protocol requested and number of scans performed.
Instruct patient to remove metal items, including eyeglasses, jewelry, hair clips, hearing aids, dentures, and clothing with zippers, buckles, or metal buttons.
Evaluate for adequate renal function if contrast will be used (see Box 15-2).
Encourage patient to remain as still as possible during the procedure.
Describe the tunnel-like narrow chamber of the closed MRI scanner and inform patient it sometimes causes feelings of anxiety or claustrophobia. Evaluate the need for sedation.
Inform patient the scanner will make a dull, thumping noise throughout the procedure.
Tell patient to resume usual activities after the procedure.
Renal insufficiency (creatinine level >1.5 or a glomerular filtration rate of <60 ml/minute).
Diabetic nephropathy and other conditions that cause reduced renal perfusion.
High total dose of contrast (<5 ml/kg or >100 ml).
Functional MRI (fMRI) is an imaging study that aids in identifying regions of the brain activated by particular stimuli and tasks. Imaging is performed during presentation of a stimulus or performance of a specific task and during rest periods. A statistical comparison is performed with images obtained during the stimulus/task periods compared with those performed during the rest periods by evaluating the conversion of oxyhemoglobin to deoxyhemoglobin and utilization of glucose, which occurs during normal brain activity. Involves IV administration of contrast material that lowers signal intensity on MRI in relation to blood flow as the material passes through the brain.
Advantages of fMRI: Does not use ionizing radiation and can be applied repeatedly in the same patient without risk. Offers potential in early detection of patients with prodromal dementia. It is also useful in preoperative evaluation of patients with lesions (tumors, seizure foci) adjacent to eloquent areas of the brain (speech center, premotor and motor cortex, and memory centers).
Disadvantages of fMRI: Same as MRI.
Instruct patient as for MRI. Full cooperation of the patient is vital for head motion and task performance reasons. Global cognitive impairment, aphasia, neglect, substantial sensory disturbances, and severe depression are usually exclusion criteria. The performance of motor tasks during imaging should be monitored.
Medications that may interfere with performance of tasks during the procedure should be avoided, if possible, including benzodiazephines, sedatives, and opioids.
Positron-emission tomography (PET) is a computer-based imaging technique that permits study of the brain’s function by evaluating the metabolism, blood flow, and chemical processes within the brain. PET measures emissions of particles of injected radioisotopes—called positrons—and converts them to an image of the brain.
PET scanners are not frequently used or widely available due to their high cost, and PET requires sophisticated equipment to produce its radioisotopes, or positron emitter.
A glucose-like solution and mildly radioactive tracers are combined for injection or inhalation. After injection into the arterial bloodstream or inhalation of this radioactive compound, pairs of gamma rays are emitted into adjacent tissue during radioactive decay. The PET scanner measures the gamma rays to determine how quickly the tissues absorb the radioactive isotopes. A computer processes the data into an image that shows where the radioactive material is located, corresponding to cellular metabolism.
Advantages of PET: Provides information on patterns of glucose and oxygen metabolism. Areas of decreased metabolism indicate dysfunction. PET is useful for early detection of Alzheimer’s disease and other dementias, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), Huntington’s disease, multiple sclerosis, and psychiatric disorders, such as depression or schizophrenia. PET can also help locate/identify abnormal brain activity, such as seizure foci, and assess brain function after stroke.
Disadvantages of PET: Ionizing radiation, high initial cost.
Inform patient that this procedure requires injection or inhalation of a radioactive substance that emits positively charged particles. Explain that the image is created when the negative particles found in the body combine with the positive particles of the imaging substance.
Explain that, after injection of the radioisotope, the patient will be asked to rest quietly on a stretcher for about 45 minutes to allow the substance to circulate.
Reassure the patient that radiation exposure is minimal.
Encourage patient to void before the test because the scan and associated procedures may take several hours.
Advise patient to increase fluid intake after the procedure to flush out the radioisotope and to resume meals.
Tell patient that it may take a few days to get results of the PET scan because it requires processing before it is available for interpretation.
SPECT is a widely available noninvasive functional imaging technique that evaluates cerebral vascular supply. A radioactive tracer is administered by inhalation or injection into the bloodstream; the tracer then decays to emit only a single photon. It uses a rotating camera to track the single photons emitted from radioactive decay and collects information from multiple views. Evaluation of the radioactive tracers creates images that show cerebral blood flow in various regions of the brain.
The radioactive tracer compounds used are commercially prepared and do not require the specialized equipment used in PET scanning.
SPECT is typically used to evaluate cerebral blood flow in patients with ischemic stroke, subarachnoid hemorrhage (SAH), migraine, dementia (including Alzheimer’s disease), epilepsy, and other degenerative diseases.
Advantages of SPECT: Can perform hemodynamic, chemical, and functional imaging; widely available.
Disadvantages of SPECT: Ionizing radiation, provides only relative measurements.
Inform patient that this is a noninvasive procedure that should cause minimal discomfort.
Tell patient that results of the scan are typically available for interpretation by a specialist immediately after the procedure.
Transcranial Doppler (TCD) ultrasonography involves noninvasive testing to measure flow changes in the form of velocities of the cerebral arteries. It helps evaluate vasospasm post-SAH, occlusion, or flow abnormalities as with stroke.
Bone windows (temporal, transorbital, or foramen magnum) are accessed using a fiber-optic probe after application of ultrasound gel. The fiber-optic probe is directed at a specific artery, and the velocities are then recorded.
Specific criteria are utilized for identification of each cerebral vessel prior to assessment of the vessel (specific bone window, depth, flow direction, and waveform).
Advantages of TCD studies: Low cost; can be performed at bedside and repeated as needed (useful in monitoring trends).
Disadvantages of TCD studies: Results are operator dependent; inability to obtain signal; limited data on sensitivity and specificity.
Explain that the study will be done with patient in a reclining position.
Inform patient that the test normally takes less than 1 hour, depending on the number of arteries that are to be studied.
CT perfusion involves rapid injection (5 to 10 mL/second) of 40 mL of iodine contrast during continuous scanning. Computer analysis of “wash-in” and “wash-out” of the contrast generates a single-slice acquisition blood flow map.
The test requires a large-bore catheter (14 or 16 gauge) for injection of the contrast. Contrast is cleared through the kidneys; therefore, renal function should be evaluated prior to study to reduce the risk of contrast-induced nephropathy (see Nursing Alert).
CT scan is programmed to analyze specific area of concern. Three 1-cm thick computer-generated images are produced that reflect relative cerebral blood flow, relative blood volume, and mean transit time.
Advantages of CT perfusion: 90% sensitivity and 100% specificity for cerebral ischemia; can be performed in conjunction with CT angiogram. Can be used in evaluating cerebral vasospasm in aneurysmal SAH, though the sensitivity and specificity is unknown.
Disadvantages of CT perfusion: Limited anatomic assessment; radiation exposure; if contrast is used there is the risk of contrast-induced nephropathy; requires large-bore catheter.
Instruct patient about the rationale for placement of largebore catheter.
Assess patient for contrast allergy and premedicate, if indicated.
Inform patient that radiation exposure, although present, is minimal.
Following local anesthesia, a radiopaque dye is injected through a catheter in the femoral artery (or brachial artery if femoral is inaccessible) and passed through one of the major cervical blood vessels to assess cerebral circulation. Serial x-rays are taken after contrast dye illuminates the cerebral arterial and venous systems. The structure and patency of cerebral arteries are examined. Contrast is cleared through the kidneys. The test is frequently performed on an outpatient basis, unless patient is already hospitalized.
Three-dimensional imaging is available for more thorough evaluation of vascular abnormalities.
Advantages of cerebral angiography: Useful in detection of stenosis or occlusion, aneurysms, and vessel displacement due to pathologic processes (eg, tumor, abscess, hematoma).
Disadvantages of cerebral angiography: Involves considerable exposure to radiation. Contraindicated in patients with a stroke in evolution. Potential complications: temporary or permanent neurologic deficit, including stroke, anaphylaxis, bleeding or hematoma at the IV site, and impaired circulation in the extremity distal to the injection site, usually the femoral artery. There is risk of contrast-induced nephropathy.
Omit the meal before the test, although clear liquids may be taken.
Evaluate for adequate renal function; should have recent normal serum creatinine.
Ask patient about allergies and specifically rule out presence of iodine allergy, which requires pretest preparation. Commonly, patients with allergy to iodine also have allergies to radiopaque contrast media that may cause severe reaction.
Options for pretest allergy prevention include:
Elective procedure: Give prednisone 50 mg orally 13, 7, and 1 hour prior to contrast and diphenhydramine 50 mg orally 1 hour prior to contrast.
Urgent procedure: Give methylprednisolone IV and diphenhydramine 50 mg orally, IM or IV 1 hour prior to contrast.
Mark pedal peripheral pulses.
Explain that a local anesthetic will be used to insert a catheter into the femoral artery (brachial artery may be used) and threaded into the required cerebral vessel.
Tell the patient to expect some discomfort when the catheter is inserted into the artery. Additionally, the sensation of a warm, flushed feeling and metallic taste should be expected when the dye is injected.
Caution the patient that he or she will need to lie still during the procedure and that he or she will be asked to hold breath intermittently during scanning.
After angiography:
Maintain bed rest and do not flex lower extremities, as ordered, and monitor vital signs. Instruct the patient to maintain bed rest for up to 6 hours. If a closing device is utilized, the time can be reduced to 2 to 3 hours.
Check the patient frequently for neurologic symptoms, such as motor or sensory alterations, reduced level of consciousness (LOC), speech disturbances, dysrhythmias, or blood pressure (BP) fluctuations.
Evaluate renal function and monitor puncture site for bleeding, hematoma, and pulses, as ordered. Apply pressure if bleeding or hematoma is noted and inform physician.
Evaluate renal function and monitor for adverse reaction to contrast medium (eg, restlessness, respiratory distress, tachycardia, facial flushing, nausea and vomiting).
Assess skin color, temperature, and peripheral pulses of the extremity distal to the IV site—change may indicate impaired circulation due to occlusion. Inform physician, if noted.
CT angiography is a minimally invasive three-dimensional (3-D) imaging technique that uses multisectional spiral CT imaging in conjunction with rapid power injection of contrast (50 mL) into a large antecubital vein. Multiple, thin slices are reconstructed to provide 3-D imaging of cerebral vasculature.
Proper timing of contrast injection with initialization of CT scans is essential to optimize intravascular enhancement.
Advantages of CT angiography: Speed of scanning allows for unstable patients to be evaluated; increasing in availability.
Disadvantages of CT angiography: Exposure to radiation; need for large-bore (18 to 20 gauge) catheter; potential for anaphylaxis; contraindicated for patients with acute/chronic renal failure.
Restrict food for 4 to 6 hours before procedure.
Assess for contrast allergy and premedicate, if indicated.
Evaluate renal function as contrast is cleared through kidneys. Elevated creatinine may preclude the individual from obtaining the study.
Magnetic resonance angiography/venography (MRA/MRV) is a 3-D phase contrast technique. The test focuses on high signal or blood flow while suppressing background nonactive tissue.
Two flow-opposing acquisitions are obtained and the computer subtracts the background signal to construct the cerebral vasculature.
Advantages of MRA/MRV: No exposure to radiation.
Disadvantages of MRA/MRV: Less sensitivity than CT angiography (sensitivity increases in aneurysms larger than 5 mm).
This scanning technique can include entire organs, such as the heart or brain, in a single rotation and enables dynamic processes, such as blood volume and time to peak, to be observed.
The scanning process takes less time and requires a smaller dose of iodinated contrast and less radiation than conventional CT, making it an ideal diagnostic test in emergency situations (heart attack or stroke) or for those who cannot tolerate large doses of iodine contrast (renal disease).
The general considerations for conventional CT are the same.
Inform the patient about lying flat on the table while the tube revolves around the area to be scanned.
If contrast will be used, the patient will most likely have an IV catheter placed for the injection of dye. A sensation of warmth or metallic taste in the mouth may be experienced after the dye injection.
A needle is inserted into the lumbar subarachnoid space, usually between the third and fourth lumbar vertebrae, and CSF is withdrawn for diagnostic and therapeutic purposes.
Purposes include:
Obtaining CSF for examination (microbiologic, serologic, cytologic, or chemical analysis).
Measuring cerebrospinal pressure and assisting in detection of obstruction of CSF circulation.
Determining the presence or absence of blood in the spinal fluid.
Aiding in the diagnosis of viral or bacterial meningitis, subarachnoid or intracranial hemorrhage, tumors, and brain abscesses.
Administering antibiotics and cancer chemotherapy intrathecally in certain cases.
Determining levels of tau protein and beta-amyloid in the CSF—a new test that may be used to assist in the diagnosis of Alzheimer’s disease. Elevated levels of tau protein and decreased levels of beta-amyloid are associated with Alzheimer’s disease.
EEG measures electrical activity of brain cells. Electrodes are attached to multiple sites on the scalp to provide a recording of electrical activity that is generated in the cerebral cortex. Electrical impulses are transmitted to an electroencephalograph, which magnifies and records these impulses as brain waves on a strip of paper. New devices allow for continuous EEG monitoring of selective areas of the brain, thus limited data is collected. It is useful in the intensive care unit (ICU) setting for continuous monitoring to evaluate for seizure activation.
Provides physiologic assessment of cerebral activity for diagnosis of epilepsy, alterations in brain activity in coma, and organic brain syndrome and sleep disorders. Particularly helpful in the investigation of patients with seizures.
Usually performed in a room designed to eliminate electrical interference; however, in the case of a comatose patient, it may be performed at bedside using a portable unit.
Restlessness and fatigue can alter brain wave patterns.
For a baseline recording, the patient is instructed to lie still and relax with eyes closed. After a baseline recording in a resting phase, the patient may be tested in various stress situations (eg, asked to hyperventilate for 3 minutes, look at a flashing strobe light) to elicit abnormal electrical patterns.
In patients with epilepsy, continuous monitoring is performed with video monitoring. This is useful for interpretation and correlation of physiologic seizure activity and clinical seizures.
Grids or strips are placed intraoperatively for more accurate assessment of seizure foci.
Mapping of seizure activity is performed to determine precise location of seizure foci.
Once foci are located, the site is evaluated for possible surgical resection.
For routine EEG, tranquilizers, anticonvulsants, sedatives, and stimulants should be held for 24 to 48 hours before the study.
Thoroughly wash and dry patient’s hair to remove hair sprays, creams, or oils.
Explain that the electrodes will be attached to patient’s skull with a special paste.
Assure patient that the electrodes will not cause shock, and encourage patient to relax during the procedure because anxiety can affect brain wave patterns.
Meals should be taken as usual to avoid sudden changes in blood glucose levels.
Sterile lumbar puncture set
Skin antiseptic (avoid use of chlorhexidine)
Sterile gloves
Adhesive bandage
Xylocaine 1%-2%
| ||||||||||||||||||||||||||||||||||||||||||||
These tests measure the brain’s electrical responses to visual, somatosensory, or auditory stimuli.
Visual evoked potentials—produced by asking the patient to look at rapidly reversing checkerboard patterns. These assist in evaluating multiple sclerosis and traumatic injury. EEG electrodes are placed over the occiput and record the transmission time from the retina to the occiput.
Somatosensory evoked potentials—generated by stimulating a peripheral sensory nerve and useful in diagnosing peripheral nerve disease and injury. These measure transmission time up the spinal cord to the sensory cortex.
Auditory evoked potentials—produced by applying sound, such as clicks, to help locate auditory lesions and evaluate integrity of the brain stem. The transmission time up the brain stem into the cortex is measured.
Can be performed intraoperatively or during interventional procedures in which patient is under general anesthesia. Acute changes may indicate potential for deficits.
Explain that the electrodes will be attached to patient’s scalp to measure the electrical activity of the nervous system. Placement of the electrodes will depend on the type of evoked potentials being measured.
Ask patient to remove all jewelry.
Assure patient that the procedure is not painful and does not cause any electric shock.
Inform patient that the test normally takes 45 to 60 minutes.
In combination with nerve conduction studies, needle electromyography (EMG) is the gold standard for assessing the neurophysiologic characteristics of neuromuscular diseases. Because it is invasive and painful, its use is limited when activity from several muscles needs to be monitored simultaneously. It is the recording of a muscle’s electrical impulses at rest and during contraction. A needle is attached to an electrode and inserted into a muscle. A mild electrical charge is delivered to stimulate the muscle at rest and during voluntary contraction. The response of the muscle is measured on an oscilloscope screen.
Useful in distinguishing lower motor neuron disorders from muscle disorders (eg, ALS from muscular dystrophy).
Nerve conduction time, another diagnostic test, is often measured simultaneously.
Explain that this test measures the electrical activity of muscles.
Advise the patient to avoid caffeine and tobacco products for 3 hours before the test, as these substances can affect test results.
Inform the patient that the procedure normally takes at least 1 hour.
Tell the patient a needle will be inserted through the skin into select muscles and to expect some degree of discomfort when the needle is inserted.
Inform the patient that, after the test, a mild analgesic or warm compresses may be needed to relieve muscle soreness.
Inform the patient to observe the needle insertion sites for bleeding, hematoma, redness, or other signs of infection and to notify the health care provider if any of these are observed.
A peripheral nerve is stimulated electrically through the skin and underlying tissues. A recording electrode detects the response from the stimulated nerve. The time between the stimulation of the nerve and the response is measured on an oscilloscope and speed of conduction along the nerve is calculated.
Used to determine lower motor neuron dysfunction, differentiating disease or injury in peripheral nerves, spinal nerve roots, or the anterior horn of the spinal cord by measuring nerve conduction velocity.
Explain that surface-stimulating electrodes with special paste are applied and taped to the nerve site (leg, arm, or face).
Advise patient that an electric current is passed through the electrode and that a mild sensation or slight discomfort may be experienced while the current is applied.
A series of tests that evaluate effects of neurologic disorders on cognitive functioning and behavior.
A neuropsychologist selects appropriate tests to determine the extent and type of functional deficits.
Paper-and-pencil tests, puzzles, and word and recall games are commonly used. Testing may assess:
Intelligence, attention span, memory, judgment.
Motor, speech, and sensory function.
Affect, coping, and adaptation.
Language quality, abstraction, distractibility.
Ability to sequence learned behaviors.
Used in diagnosis of organic brain dysfunction and dementia.
Valuable in determining vocational rehabilitation training needs.
Utilized in some facilities to determine competency of a patient with regard to instituting durable power of attorney or need for guardianship.
Assure patient that these tests are not intended to evaluate mental illness.
Explain that testing evaluates the ability to remember, calculate numbers, and perform abstract reasoning.
Patient should be well rested because testing is mentally tiring and lengthy. A complete examination is a 4- to 6-hour process, depending on patient’s ability to concentrate.
Anticipate fatigue and frustration after the examination.
Polysomnography is a noninvasive, all-night sleep study that measures character of sleep, simultaneously monitoring EEG, cardiac and respiratory function, and movements during sleep. It is used to confirm fragmented sleep patterns in narcolepsy and sleep-related epilepsy.
Testing is time-consuming and labor-intensive. Procedures typically include multiple physiologic measures, such as EEG, EMG, electrocardiogram [ECG]), heart rate, respiratory effort, air flow, and oxygen saturation.
Explain that the electrodes placed on the scalp, chest, extremities, and face will be uncomfortable but do not deliver electrical current.
Reassure patient that a technician will be in the next room.
Advise patient to wear comfortable nightwear.
Multisleep latency test (MSLT) is a sleep study performed during the day. It is the most widely objective assessment of daytime sleepiness and is commonly used to confirm a diagnosis of narcolepsy.
Testing consists of four “napping” periods of 20 to 35 minutes, during which time the patient lies down on a bed in a darkened room and is allowed to fall asleep. The multiple short sleep periods during the MSLT increase the observation of rapid-eye-movement (REM) periods.
Explain the time and duration of the naps.
Reassure patient of freedom to move about between naps.
Tell patient to wear comfortable clothing and to bring reading or other materials for use between naps.
Assess eye opening (level of responsiveness).
Eye opening = arousal
Tracking = awareness
Assess neurologic function using the GCS. The GCS addresses eye opening, verbal responses, and motor responses. If the patient’s eyes do not open spontaneously or to your voice, then assess responses using painful stimuli by applying pressure against the nail bed, trapezius/axillary pinch, or sternum. Use the least amount of pain for the best response.
Assess cognitive function.
Orientation.
Person, place, and time.
Where are you, why are you here.
General information—national and local current events.
Speech—aphasia and other problems (see Table 15-3).
Fluent aphasia (motor/Broca’s)—inability to express self.
Nonfluent aphasia (sensory/Wernicke’s)—inability to understand the spoken language.
Global aphasia—inability to speak or understand spoken language.
Other aphasia syndromes—amnesia, conduction.
Other alterations include:
Confabulation—fluent, nonsensical speech.
Preservation—continuation of thought process with inability to change train of thought without direction or repetition.
Assess motor function—voluntary versus reflexive:
Voluntary movement.
Normal complex movement—strength and symmetry in the upper extremities (UE), pronator drift proximally and grip strength distally; in the lower extremities (LE), leg lifts proximally and dorsi/plantar flexion distally.
Localization—ability to determine location of stimuli; patient localizes area of painful stimuli.
Withdrawal—abduction of the upper extremity; moving away from the stimuli.
Reflexive movement.
Abnormal flexor posturing (decorticate)—dysfunction of corticospinal tracts above the brain stem. Abnormal flexion of the UE with adduction of the UE, internal rotation of the upper extremity, wrist and extension, internal rotation and plantar flexion of the LE.
Abnormal extension posturing (decerebrate)—dysfunction of vestibulospinal tract and the RAS of the upper brain stem. Abnormal extension, hyperpronation, and adduction of the UE and wrist flexion; abnormal extension and internal rotation of the LE with plantar flexion of the feet and toes.
Mixed posturing—varied extensor and flexor tone in UE.
Flaccid—medullary compression with complete loss of motor tone.
Test cranial nerve (CN) reflexes to assess for brain stem dysfunction.
Assess pupil size, symmetry, and reaction to light.
Assess extraocular movements (CNs III, IV, VI) and reflex eye movements elicited by head turning (oculocephalic response). This should not be performed on patients with suspected cervical spine injury, patients in a cervical collar, or patients known to have cervical spine injuries.
The oculovestibular (caloric) response (CNs III, IV, VI, VIII) is tested by medical staff when the patient is comatose and the oculocephalic response is absent, as a determination of brain death.
Assess CNs V and VII together to evaluate facial pain, blink, eye closure, and grimace.
Assess CNs IX, X, XII to evaluate gag, swallowing reflex, tongue protrusion, and patient’s ability to handle own secretions.
Assess respiratory rate and pattern (normal, Kussmaul, Cheyne-Stokes, apneic).
Assess deep tendon reflexes; evaluate tone for spasticity, rigidity, and paratonia (abnormal resistance increasing throughout flexion and extension, indicating frontal lobe dysfunction).
Examine head for signs of trauma, and mouth, nose, and ears for evidence of edema, blood, and CSF (may indicate basilar skull fracture).
Monitor any change in neurologic status over time and report changes to health care provider, as indicated.
 Key Decision Point
Key Decision PointTable 15-2 Glasgow Coma Scale | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Table 15-3 Differentiating Aphasia | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Decreased Intracranial Adaptive Capacity.
Ineffective Airway Clearance related to upper airway obstruction by tongue and soft tissues, inability to clear respiratory secretions.
Risk for Imbalanced Fluid Volume related to inability to ingest fluids, dehydration from osmotic therapy (when used to reduce intracranial pressure [ICP]).
Impaired Oral Mucous Membranes related to mouth breathing, absence of pharyngeal reflex, inability to ingest fluid.
Risk for Impaired Skin Integrity related to immobility or restlessness.
Impaired Tissue Integrity of cornea related to diminished/absent corneal reflex.
Hyperthermia related to infectious process; damage to hypothalamic center.
Impaired Urinary Elimination related to unconscious state.
Bowel Incontinence related to unconscious state.
Monitor for change in neurologic status, decreased LOC, onset of CN deficits.
Identify emerging trends in neurologic function and communicate findings to medical staff.
Monitor response to pharmacologic therapy, including drug levels, as indicated.
Monitor laboratory data, CSF cultures, and Gram stain, if applicable, and communicate findings to medical staff.
Assess neurologic drains/dressings for patency, security, and characteristics for drainage.
Institute measures to minimize risk for increased ICP, cerebral edema, seizures, or neurovascular compromise.
Adjust care to reduce risk of increasing ICP: body positioning in a neutral position (head aligned with shoulders) without flexing head, reduce hip flexion, distribute care throughout 24-hour period sufficiently for ICP to return to baseline.
Monitor temperature status; maintain normothermia. Institute cooling procedure as ordered.
Position patient to prevent tongue from obstructing the airway, encourage drainage of respiratory secretions, and promote adequate exchange of oxygen and carbon dioxide.
Keep the airway free from secretions with suctioning. In the absence of cough and swallowing reflexes, secretions rapidly accumulate in the posterior pharynx and upper trachea and can lead to respiratory complications (eg, aspiration).
Insert oral airway if tongue is paralyzed or is obstructing the airway. An obstructed airway increases ICP. This is considered a short-term measure.
Prepare for insertion of cuffed endotracheal tube to protect the airway from aspiration and to allow efficient removal of tracheobronchial secretions.
See page 231 for technique of tracheal suctioning.
Use oxygen therapy as prescribed to deliver oxygenated blood to the CNS.
Before suctioning, pretreat with sedative, opioid, or endotracheal lidocaine, if indicated.
Monitor prescribed IV fluids carefully, maintaining euvolemia and minimizing large volumes of “free water,” which may aggravate cerebral edema.
Maintain hydration and enhance nutritional status with use of enteral or parenteral fluids.
Measure urine output.
Evaluate pulses (radial, carotid, apical, and pedal) and measure BP; these parameters are a measure of circulatory adequacy/inadequacy.
Maintain circulation; support the BP and treat life-threatening cardiac dysrhythmias.
Remove dentures. Inspect patient’s mouth for dryness, inflammation, and the presence of crusting.
Provide mouth care by brushing teeth and cleansing the mouth with appropriate solution every 2 to 4 hours to prevent parotitis (inflammation of parotid gland).
Apply lip emollient to maintain hydration and prevent dryness.
Keep the skin clean, dry, well lubricated, and free from pressure because comatose patients are susceptible to the formation of pressure ulcers.
Turn the patient from side to side on a regular schedule to relieve pressure areas and help clear lungs by mobilizing secretions; turning also provides kinesthetic (sensation of movement), proprioceptive (awareness of position), and vestibular (equilibrium) stimulation.
Reposition carefully after turning to prevent ischemia and shearing over pressure areas.
Position extremities in functional position, and monitor skin underneath splints/orthosis to prevent skin breakdown and pressure neuropathies.
Perform range-of-motion (ROM) exercises of extremities at least four times daily; contracture deformities develop early in unconscious patients.
Protect the eyes from corneal irritation as the cornea functions as a shield. If the eyes remain open for long periods, corneal drying, irritation, and ulceration are likely to result.
Make sure the patient’s eye is not rubbing against bedding if blinking and corneal reflexes are absent.
Inspect the condition of the eyes with a penlight.
Remove contact lenses, if worn.
Irrigate eyes with sterile saline or prescribed solution to remove discharge and debris.
Instill prescribed ophthalmic ointment in each eye to prevent glazing and corneal ulceration.
Apply eye patches, when indicated, ensuring that eyes remain closed under patch.
Prepare for temporary tarsorrhaphy (suturing of eyelids in closed position) if unconscious state is prolonged.
Look for possible sites of infections (respiratory, CNS, urinary tract, wound) when fever is present in an unconscious patient.
Monitor temperature frequently or continuously.
Control persistent elevations of temperature. Fever increases metabolic demands of the brain, decreases circulation and oxygenation, and results in cerebral deterioration.
Monitor core temperature continuously and treat hyperthermia promptly. Hyperthermia increases the brain’s metabolic rate and increases the risk of secondary injury. A body core temperature is 4 to 5 degrees lower than brain temperature.
Maintain a cool ambient temperature. Anticipate potential for overcooling and make environmental adjustments accordingly (eg, operating room environment).
Minimize excess covering on bed.
Administer prescribed antipyretics.
Use cool-water sponging and an electric fan blowing over the patient to increase surface cooling for hyperthermia resistant to antipyretics.
Use an external cooling device to maintain normothermia, but avoid rapid overcooling. Intravascular cooling devices may also be used.
An indwelling or external urethral catheter may be used for short-term management.
Use intermittent bladder catheterization for distention as soon as possible to minimize risk of infection. Palpate over the patient’s bladder at intervals or use a bladder scan to detect urine retention and an overdistended bladder.
Monitor for fever and cloudy urine.
Initiate a bladder-training program as soon as consciousness is regained.
Auscultate for bowel sounds; palpate lower abdomen for distention.
Observe for constipation due to immobility and lack of dietary fiber. Stool softener or laxative, scheduled or as needed, may be prescribed to promote bowel elimination. The goal is bowel movement every other day.
Monitor for diarrhea resulting from infection, antibiotics, enteric feedings, hyperosmolar fluids, and fecal impaction.
Perform a rectal examination if fecal impaction is suspected.
Use fecal collection bags and provide meticulous skin care if patient has fecal incontinence.
Develop a supportive and trusting relationship with the family or significant other.
Provide information and frequent updates on the patient’s condition and progress.
Involve them in routine care and teach procedures that they can perform at home.
Demonstrate and teach methods of sensory stimulation to be used frequently.
Use physical touch and reassuring voice.
Talk to patient in a meaningful way even when the patient does not seem to respond. Assume patient is able to hear even if unresponsive.
Orient the patient periodically to person, time, and place.
Demonstrate and teach methods frequently used to manage restlessness/agitation.
Eliminate distractions.
Reduce environmental stimuli (turn off television and radio, close door).
Use one-to-one communication.
Talk slowly and simplify information without talking down to the person.
Teach the family to recognize and report unusual restlessness, which could indicate cerebral hypoxia, metabolic imbalance, or pain.
Enlist the help of the social worker, home health agency, or other resources to assist family with such issues as financial concerns, guardianship, need for additional follow-up care (rehabilitation, long-term care facility), need for medical equipment in home, and/or respite care.
Neurologic status remains at baseline or improved.
Maintains clear airway; coughs up secretions.
Absence of signs of dehydration.
Intact, pink mucous membranes.
No skin breakdown or erythema.
Absence of trauma to cornea.
Core temperature within normal limits.
Absence of urinary tract infection (UTI); maintenance of normal bladder emptying.
Bowel movement on regular basis in response to bowel regimen.
 Evidence Base
Evidence Base
ICP is comprised of the following components and volume ratio: brain tissue, 80%; CSF, 10%; blood volume, 10%.
The Monroe-Kellie doctrine states that the intracranial vault is a closed structure with a fixed intracranial volume. The intracranial contents must be kept in equilibrium and the ratio between volume and pressure must remain constant. Any increase in the volume of one component must be accompanied by a reciprocal decrease in one of the other components. When this volume-pressure relationship becomes unbalanced, ICP increases.
The brain attempts to compensate for rises in ICP by:
Displacement/shunting of CSF from the intracranial compartment to the lumbar subarachnoid space (SAS). Normally, about 500 mL of CSF are produced and absorbed in 24 hours. About 125 to 150 mL circulates throughout the ventricular system and the SAS in the following ratio: 25 mL in the ventricles, 90 mL in the lumbar SAS, and 35 mL in the cisterns and surrounding SAS.
Increased CSF absorption.
Decreased cerebral blood volume by displacement of cerebral venous blood into the venous sinuses. Compensatory measures are finite. Increased ICP will ultimately occur if the volume of the intracranial mass exceeds the volume compensated.
Intracranial compliance is “tightness” of the brain. Compliance is the relationship between intracranial volume and ICP. It is a nonlinear relationship; as ICP increases, compliance decreases. With functional compensatory mechanisms, an increase in volume causes a small, transient increase in ICP. As compliance decreases, small increases in volume result in moderate increases in pressure. When compensatory mechanisms are exhausted, very slight increases in volume will produce large increases in pressure. The patient’s response to changes in ICP will depend on where the patient is on the volume-pressure curve.
Factors that influence the ability of the body to achieve this steady state include:
Systemic BP.
Ventilation and oxygenation.
Metabolic rate and oxygen consumption (fever, shivering, physical activity).
Regional cerebral vasospasm.
Oxygen saturation and hematocrit.
Inability to maintain a steady state results in increased ICP. Traumatic brain injury, cerebral edema, intracerebral hemorrhage, ischemic stroke, abscess and infection, lesions, intracranial surgery, and radiation therapy can be potential etiologies of increased ICP.
Increased ICP constitutes an emergency and requires prompt treatment. ICP can be monitored by means of an intraventricular catheter, a subarachnoid screw or bolt, or an epidural pressure recording device.
Alterations or compromise in cerebral blood flow can be measured noninvasively by a TCD study. Increased velocities indicate vasospasm, diminished velocities indicate low blood flow, and absent velocities are consistent with no flow or brain death.
Change in LOC (awareness): drowsiness, lethargy.
Early behavioral changes: restlessness, irritability, confusion, and apathy.
Falling score on the GCS (see page 483).
Change in orientation: disorientation to time, place, or person.
Difficulty or inability to follow commands.
Difficulty or inability in verbalization or in responsiveness to auditory stimuli.
Change in response to painful stimuli (eg, purposeful to inappropriate or absent responses).
Posturing (abnormal flexion or extension).
Rising BP or widening pulse pressure (the difference between systolic and diastolic BP). This may be followed by hypotension and labile vital signs, indicating further brain stem compromise.
Pulse changes with bradycardia changing to tachycardia as ICP rises.
 Key Decision Point Watch for Cushing’s triad—bradycardia, hypertension (with widening pulse pressure), and irregular respirations; this is classic symptomatology related to uncompensated increased ICP and is considered a neurologic medical emergency. Alert health care provider and prepare for therapeutic intervention.
Key Decision Point Watch for Cushing’s triad—bradycardia, hypertension (with widening pulse pressure), and irregular respirations; this is classic symptomatology related to uncompensated increased ICP and is considered a neurologic medical emergency. Alert health care provider and prepare for therapeutic intervention.
Respiratory irregularities: tachypnea (early sign of increased ICP); slowing of rate with lengthening periods of apnea; Cheyne-Stokes (rhythmic pattern of increasing and decreasing depth of respirations with periods of apnea) or Kussmaul (paroxysms of difficult breathing) breathing; central neurogenic hyperventilation (prolonged, deep breathing); apneustic (sustained inspiratory effort) breathing; and ataxic (incoordinated and spasmodic) breathing.
Hyperthermia followed by hypothermia.
Caused by increased pressure on optic and oculomotor nerves.
Inspect the pupils with a penlight to evaluate size, configuration, and reaction to light.
Compare both eyes for similarities or differences, particularly pupillary changes related to location and progression of brain stem herniation.
Midbrain involvement—fixed and dilated.
Pontine involvement—pinpoint pupils.
Uncal herniation.
Unilaterally dilating pupil ipsilateral to lesion.
Anisocoria (unequal) with sluggish light reaction in dilated pupil.
If treatment is delayed or unsuccessful, contralateral pupil becomes dilated and fixed to light.
When herniation of brain stem occurs, both pupils assume midposition and remain fixed to light.
Central transtentorial herniation.
Pupils are small bilaterally (1 to 3 mm).
Reaction to light is brisk but with small range of constriction.
If treatment is delayed or unsuccessful; small pupils dilate moderately (3 to 5 mm) and fix irregularly at midposition.
When herniation of brain stem occurs, both pupils dilate widely and remain fixed to light.
Perform funduscopic examination to inspect the retina and optic nerve for hemorrhage and papilledema.
Evaluate gaze to determine if it is conjugate (paired, working together) or dysconjugate (eye deviates or movement is asymmetric).
Evaluate movement of eyes.
Inability to abduct or adduct: deviation of one or both eyes.
Alteration in vision (eg, blurred vision, diplopia, field cut).
Spontaneous roving, random eye movements.
Nystagmus on horizontal/vertical gaze.
Oculocephalic reflex (doll’s eyes): brisk turning of the head left, right, up, or down with observation of eye movements in response to the stimulus. Tests brain stem pathways between CNs III, IV, VI, and VIII. This should not be performed on patients with suspected cervical spine injury, patients in a cervical collar, or patients with known cervical spine injuries unless part of the brain death exam.
Oculovestibular reflex (cold calorics): 30 to 60 mL of ice water instilled into the ear with the head of the bed elevated to 30 degrees. Tests brain stem pathways between CNs III, IV, VI, and VIII. Response preserved longer than the doll’s eyes maneuver. Performed by health care provider as part of brain death examination.
Headache increasing in intensity and aggravated by movement and straining.
Vomiting recurrent with little or no nausea, especially in early morning; may be projectile.
Papilledema from optic nerve compression.
Subtle changes, such as restlessness, headache, forced breathing, purposeless movements, and mental cloudiness.
Motor and sensory dysfunctions (proximal muscle weakness, presence of pronator drift).
Contralateral hemiparesis progressing to complete hemiplegia.
Speech impairment (nonfluent, fluent, or global aphasia) when dominant hemisphere involved.
Seizure activity: focal or generalized.
Decreased brain stem function (CN deficits, such as loss of corneal reflex, gag reflex, and ability to swallow).
Pathologic reflexes: Babinski, grasp, chewing, sucking.
Decreased Intracranial Adaptive Capacity.
 NURSING ALERT
NURSING ALERT
Establish and maintain airway, breathing, and circulation.
Promote normal Pco2. Hyperventilation is not recommended for prophylactic treatment of increased ICP as cerebral circulation is reduced by 50% the first 24 hours after injury. Hyperventilation causes cerebral vasoconstriction and decreases cerebral blood flow to decrease ICP; this can potentiate secondary injury to the brain. Hyperventilation should be used only after all other treatment options have been exhausted or in an acute crisis.
Avoid hypoxia. Decreased PO2 (less than 60 mm Hg) causes cerebral vasodilation, thus increasing ICP.
Maintain cerebral perfusion pressure (CPP) greater than 50 mm Hg. CPP is determined by subtracting the ICP from the MAP: CPP = MAP – ICP.
Administer mannitol (0.25 to 1 g/kg), an osmotic diuretic, if ordered. Osmotic diuretics act by establishing an osmotic gradient across the blood-brain barrier that depletes the intracellular and extracellular fluid volume within the brain and throughout the body. Mannitol will be ineffective if the blood-brain barrier is not intact.
Administer hypertonic saline (2% or 3%), if ordered. It creates an osmotic gradient that pulls extra fluid from the brain with an intact blood-brain barrier, lowers ICP, improves cerebral blood flow by reducing viscosity, and improves oxygen carrying capacity. Saline (23.4%) is used as a bolus to treat acute increases in ICP in conjunction with or in place of mannitol.
Insert an indwelling urinary catheter for management of diuresis.
Administer corticosteroids, such as dexamethasone, as ordered, to reduce vasogenic edema associated with brain tumors. Corticosteroids are not recommended in the treatment of cytoxic (intracellular) cerebral edema related to trauma or stroke.
Maintain balanced fluids and electrolytes. Watch for increased or decreased serum sodium due to the following conditions that may occur with increased ICP.
Diabetes insipidus (DI) results from the absence of antidiuretic hormone (ADH); this is reflected by increased urine output with elevation of serum osmolarity and sodium.
The syndrome of inappropriate antidiuretic hormone (SIADH) results from the secretion of ADH in the absence of changes in serum osmolality. This is a hypervolemic state reflected by decreased urine output with decreased serum sodium and increased free water.
Cerebral salt wasting is associated with abnormal release of aldosterone resulting in increased elimination of sodium and decreased interstitial volume (hypovolemic state) (see Table 15-4).
Monitor effects of anesthetic agents, such as propofol, and sedatives, such as midazolam, which may be given to prevent sudden changes in ICP due to coughing, straining, or “fighting” the ventilator. Short-acting medications are preferred to allow for intermittent neurological assessment.
High-dose barbiturates, such as pentobarbital, may be used in patients with refractory increased ICP. (Note: Prophylactic use is not recommended. It is utilized when all other treatments have failed.) Dosing: 10 mg/kg over 30 minutes; then 5 mg/kg every hour for 3 hours followed by maintenance dose of 1 mg/kg/hr. (Goal serum barbiturate level of 3 to 4 mg/dL.)
High-dose barbiturates induce a comatose state and suppress brain metabolism, which, in turn, reduces cerebral blood flow and ICP. Only pupillary response is assessed.
Be alert to the high level of nursing support required. All responses to environmental and noxious stimuli (suctioning, turning) are abolished as well as all protective reflexes.
Cough or gag reflex will be absent and the patient will be unable to protect the airway, increasing susceptibility to pneumonia.
Monitor ICP, arterial pressure, and serum barbiturate levels, as indicated. Perform continuous EEG monitoring to document burst suppression (suppression of cortical activity) and ensure adequate dosing of barbiturates, if used.
Monitor temperature because barbiturate coma causes hypothermia.
Diminished GI motility and high risk for ileus.
Maintain normothermia and treat fever aggressively. Fever increases cerebral blood flow and cerebral blood volume; acute increases in ICP occur with fever spikes. Cerebral temperature is 4 to 5 degrees higher then body core temperature; therefore, small increases in body core temperature can create drastic increases in the core temperature of the brain. Infection is a common complication of ICP, and in the presence of fever, an infectious workup should be completed.
Avoid positions or activities that may increase ICP. Keep head in alignment with shoulders; neck flexion or rotation increases ICP by impeding venous return. Keep head of bed elevated 30 degrees to reduce jugular venous pressure and decrease ICP.
Minimize suctioning, keep procedure less than 15 seconds, and, if ordered, instill lidocaine via endotracheal (ET) tube before suctioning. Coughing and suctioning are associated with increased intrathoracic pressure, which is associated with ICP spikes. Inject 5 to 10 mL of lidocaine into ET tube before suctioning to dampen the cough response.
Minimize other stimuli, such as alarms, television, radio, and bedside conversations, which may precipitously increase ICP (stimuli that create elevation in ICP are patient-dependent).
Maintain normal blood sugar levels. Treat with sliding scale insulin or insulin drip as ordered.
Initiate treatment modalities, as ordered, for increased ICP (above 20 mm Hg or if there is a significant shift in pressure).
Pretreat prior to known activities that raise ICP and avoid taking pressure readings immediately after a procedure. Allow patient to rest for approximately 5 minutes.
Record ICP readings every hour and correlate with significant clinical events or treatments (suctioning, turning).
Table 15-4 Differentiating Etiology of Hyponatremia | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
ICP and vital signs stable; alert and responsive.
 Evidence Base
Evidence Base
External ventricular drain (EVD): catheter is inserted into lateral ventricle (right is preferred) through a drilled burr hole opening; connected to fluid-filled transducer, which converts mechanical pressure to electrical impulses and waveform; allows ventricular drainage. EVD is the most accurate method to measure ICP.
Subarachnoid (bolt) hollow screw inserted into SAS beneath skull and dura through drill hole; also connected to pressure transducer system.
Epidural sensor inserted beneath skull but not through dura, so does not measure pressure directly; fiber-optic cable is connected directly to monitor.
Parenchymal device is inserted directly into brain tissue.
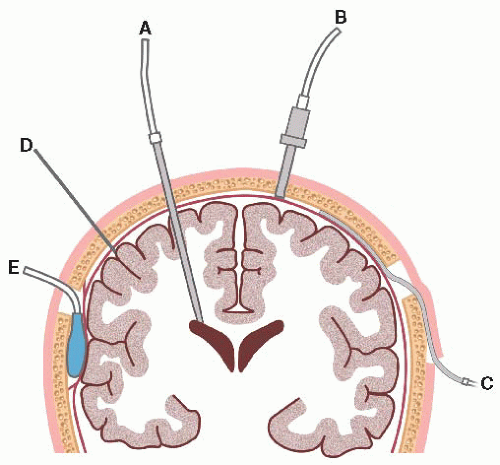 Figure 15-1. Intracranial pressure monitoring system. (A) Intraventricular; (B) subarachnoid; (C) subdural; (D) parenchymal; (E) epidural. |
Sterile gloves, mask, and surgical cap
Monitoring system (intraventricular, subarachnoid, epidural)
IV pole or stand on which to mount the system
IV solutions, as ordered
IV high-pressure tubing
Burr hole tray for insertion or as needed
Topical anesthetic
Vital sign records
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
The normal pulse waveform consists of three identifiable components: P-1, P-2, and P-3 (see diagram A on page 491).
P-1, the percussive wave, reflects pulsations of the choroid plexus, where CSF is produced, as transmitted from the cardiovascular system at systole. It is the highest of the three waveform components under normal conditions.
P-2, the tidal wave, has a more variable shape and reflects relative brain volume. P-2 can become elevated in response to a mass lesion or when brain compliance is decreased.
P-3, the dicrotic wave, follows the dicrotic notch on the downslope of the individual ICP pulse waveform and is usually the lowest waveform segment.
Plateau or A wave patterns are pathological, reflecting a rapid rise in ICP up to 50 to 100 mm Hg, and may be followed by a variable period during which the ICP remains elevated and then falls to baseline. Truncated patterns that do not exceed an elevation of 50 mm Hg are early indicators of neurological deterioration. A waves are clinically significant as elevation in ICP is related to compromise in autoregulation secondary to increased cerebral blood volume and decreased cerebral blood flow.
B and C wave patterns are related to respiration and are of little clinical significance. B wave patterns are of shorter duration and smaller amplitude than A wave patterns and may precede A wave patterns. C wave patterns are small, rhythmic oscillations that fluctuate with changes in respiration and BP.
Licox Brain Oxygen Monitoring System—placed in the brain tissue through a burr hole and monitors brain tissue partial pressure of oxygen (PbrO2), cerebral temperature, and indirect ICP. Continual monitoring of the cerebral temperature and oxygenation levels provides direct information on the acute changes in the intracranial tissue that can potentiate secondary brain injury.
Microdialysis—catheter is placed into the brain tissue through a burr hole for monitoring of cerebral oxygen, glucose, lactate, lactate-pyruvate, glutamate, and glycerol. The catheter is connected to a 2.5-mL syringe and into a microinfusion pump. The pump is perfused with Ringer’s solution. Samples are obtained periodically for analysis.
Jugular venous oximetry—A fiber-optic oximetric catheter is placed into the jugular bulb of the internal jugular vein for measurement of jugular venous oxygen saturation (SjvO2). SjvO2 is helpful in evaluating arterial saturation, cerebral metabolic rate for oxygen, and cerebral blood flow. The normal range is between 54% and 75%. A low SjvO2 is suggestive of increased brain extraction of oxygen related to systemic arterial hypoxia, decreased cerebral blood flow from hypotension or vasospasm, or an elevated ICP with a low CPP. Desaturation is thought to be associated with ischemic events and directly related to increased morbidity.
Note the pattern of waveforms and any sustained elevation of pressure above 20 mm Hg.
 NURSING ALERT The frequency of brief elevations in ICP > 20 mm Hg and CPP < 50 mm Hg correlates with worsening outcomes following traumatic brain injury. Measures to reduce increased ICP should be performed immediately and the health care provider notified when the ICP remains elevated >5 minutes.
NURSING ALERT The frequency of brief elevations in ICP > 20 mm Hg and CPP < 50 mm Hg correlates with worsening outcomes following traumatic brain injury. Measures to reduce increased ICP should be performed immediately and the health care provider notified when the ICP remains elevated >5 minutes.
Avoid overstimulation of the patient.
Note the stimuli that cause increased pressure, such as bathing, suctioning, repositioning, or visitors. Adjust care, as indicated.
Premedicate, as indicated.
Provide rest periods between periods of care.
Limit visitors as status indicates.
Limit unnecessary conversation at patient’s bedside.
Eliminate external environmental stimuli. Close doors, turn off suction equipment when not in use, limit television or radio as status indicates.
Watch for developing or increasing P-2 waves and frequency of A (plateau) waves. Report these, and begin measures to lower increased ICP, as described on page 491.
Diagnostic findings, surgical procedure, and expectations are reviewed with the patient.
Presurgical shampoo with an antimicrobial agent may be ordered. Skull preparation is performed in the operating room.
Depending on primary diagnosis, corticosteroids may be ordered preoperatively to reduce vasogenic cerebral edema.
Depending on the type and location of lesion, anticonvulsants may be ordered to reduce risk of seizures.
The patient is prepared for the use of intraoperative antibiotics to reduce risk of infection.
Urinary catheterization is performed to assess urinary volume during operative period.
If cerebral edema develops, intraoperative or postoperative osmotic diuretic (mannitol) or corticosteroids may be ordered for its treatment.
Neurologic assessment is performed to evaluate and record the patient’s neurologic baseline and vital signs for postoperative comparison.
Family and patient are made aware of the immediate postoperative care and where the physician will contact the family after surgery.
Supportive care is given, as needed, for neurologic deficits.
Respiratory status is assessed by monitoring rate, depth, and pattern of respirations. A patent airway is maintained.
Vital signs and neurologic status are monitored using a facility-based neurological assessment tool; findings are documented. Arterial line may be used for blood pressure monitoring.
Pharmacologic agents may be prescribed to control increased ICP.
Incisional and headache pain may be controlled with analgesic such as an opioid or acetaminophen, as prescribed. Monitor response to medications.
Position head of bed at 15 to 30 degrees, or per clinical status of the patient, to promote venous drainage. Determining appropriate position of head of bed is patient-dependent and should be adjusted based on observed changes in the patient’s clinical response and ICP to positioning. A decrease in CPP is observed with raising the head of the bed to lower ICP.
Turn side-to-side every 2 hours; positioning restrictions will be ordered by the health care provider (craniectomy patients should not be turned on the side of the cranial defect).
CT scan of the brain is performed if the patient’s status deteriorates.
Oral fluids are provided when the patient is alert and swallow reflex has returned. Intake and output are monitored. Speech therapy may be ordered for bedside swallow study or radiographic swallow study.
Signs of infection are monitored by checking craniotomy site, ventricular drainage, nuchal rigidity, or presence of CSF (fluid collection at surgical site).
Periorbital edema is controlled by such measures as elevation of head of bed and cold compresses. Removal of surgical dressing and increase in activity will assist in the resolution of periorbital edema.
Intracranial hemorrhage/hematoma.
Cerebral edema.
Infections (eg, postoperative meningitis, pulmonary, wound).
Seizures.
CN dysfunction.
Decreased CPP causing cerebral ischemia.
Risk for Ineffective Cerebral Tissue Perfusion related to increased ICP.
Risk for Aspiration related to decreased swallow reflex and postoperative positioning.
Risk for Infection related to invasive procedure.
Acute Pain related to surgical wound.
Constipation related to use of opioids and immobility.
Closely monitor LOC, vital signs, pupillary response, and ICP, if indicated. Notify health care provider if ICP is greater than 20 mm Hg or CPP is less than 50 mm Hg.
Teach the patient to avoid activities that can raise ICP, such as excessive flexion or rotation of the head and Valsalva maneuver (coughing, straining with defecation).
Administer medications, as prescribed, to reduce ICP.
Eliminate noxious tactile stimuli, such as suctioning, prolonged physical assessment, turning, and ROM exercises (based on patient response).
Offer fluids only when the patient is alert and swallow reflexes have returned.
Have suction equipment available at bedside. Suction only if indicated. Pretreat with sedation or endotracheal lidocaine to prevent elevation of ICP.
Elevate head of bed to maximum of order, or per clinical status, and patient comfort.
Use sterile technique for dressing changes, catheter care, and ventricular drain management.
Be aware of patients at higher risk of infection—those undergoing lengthy operations, those with ventricular drains left in longer than 72 hours, and those with operations of the third ventricle.
Assess surgical site for redness, tenderness, and drainage.
Watch for leakage of CSF, which increases the danger of meningitis.
Watch for sudden discharge of fluid from wound; a large leak usually requires surgical repair.
Warn against coughing, sneezing, or nose blowing, which may aggravate CSF leakage.
Assess for moderate elevation of temperature and neck rigidity.
Note patency of ventricular catheter system.
Institute measures to prevent respiratory or UTI postoperatively.
Encourage fluids when patient is able to manage liquids.
Ambulate as soon as possible.
Change to nonopioid agents for pain control as soon as possible.
Avoid Valsalva-like maneuvers.
Use stool softeners and laxatives, as ordered.
Keep patient and family aware of progress and plans to transfer to step-down unit, general nursing unit, subacute care, or rehabilitation facility.
Encourage frequent visiting and interaction of family for stimulation of patient as care allows.
Begin discharge planning early, and obtain referral for home care nursing, social work, or physical and occupational therapy, as needed.
Normal ICP and CPP maintained between 50 and 70 mm Hg.
Gag reflex present; breath sounds clear.
Afebrile without signs of infection.
Verbalizes decreased pain.
Passed soft stool.
Cause is unknown. Possible etiologies include sensory ganglionitis of the CNS with secondary muscle palsy, caused by inflammation, vascular ischemia, and autoimmune demyelination.
Most patients experience a viral prodrome (eg, upper respiratory infection, herpes simplex virus) 1 to 3 weeks before onset of symptoms.
Can affect anyone at any age; however, it disproportionately affects pregnant women and those who have diabetes or influenza.
Generally self-limiting. With or without treatment, most patients improve significantly within 2 weeks and about 80% recover completely within 3 months. In rare cases, the symptoms may never completely resolve or may recur. Risk factors thought to be associated with a poor outcome include: (1) over age 60 years, (2) complete paralysis, and (3) decreased taste or salivary flow on the side of paralysis.
Acute onset of unilateral upper and lower facial paralysis (over a 48-hour period). Paralysis of ipsilateral side of face from vertex of scalp to chin; facial muscles weak throughout forehead, cheek, and chin; can affect speech and taste, distort face, decrease tearing, and cause posterior auricular pain.
Involvement of all branches of facial nerve: facial weakness, diminished taste from anterior two thirds of tongue, decreased blink reflex, decreased lacrimation, inability to close eye, painful eye sensations, photophobia, drooling.
Hyperacusis on the affected side.
History to determine previous illness, onset of paralysis, and associated symptoms.
Exclusion of lesions that mimic Bell’s palsy, such as tumor, infection (Lyme disease, meningitis), trauma, stroke, or other conditions (sarcoidosis, multiple sclerosis, Guillain-Barre syndrome) through thorough neurologic examination and CT scan.
Electrophysiologic testing, specifically action potentials, EMGs, and nerve conduction velocities, to evaluate nerve function.
Lyme disease serological testing history of tick bite or bilateral weakness. About 5% to 10% of untreated Lyme disease patients may develop Bell’s palsy.
 NURSING ALERT
NURSING ALERT
Corticosteroid therapy may be initiated early to decrease inflammation (eg, prednisone 1 mg/kg/day for 10 to 14 days, followed by a tapering dose). Acyclovir combined with prednisone may improve facial function. When using corticosteroids for the treatment of Bell’s palsy, caution should be used in patients with tuberculosis, peptic ulcer disease, diabetes mellitus, renal or hepatic dysfunction, or malignant hypertension. For patients who have a contraindication to steroid therapy, acyclovir may be given as solitary treatment.
Eye care to maintain lubrication and moisture if unable to close. May need to be patched during sleep.
Physical therapy, electrical stimulation to maintain muscle tone.
Biofeedback as adjunct therapy.
Surgery to anastomose facial nerve to other cranial nerve (CN VII to XI or CN VII to XII; although Bell’s palsy is usually self-limiting); surgical closure of eyelid to protect cornea (tarsorrhaphy).
Corneal ulceration.
Impairment of vision.
Psychosocial adjustment to prolonged paralysis.
 NURSING ALERT
NURSING ALERT
Test motor components of facial nerve (VII) by assessing patient’s smile, ability to whistle, purse lips, wrinkle forehead, and close eyes. Observe for facial asymmetry.
Observe patient’s ability to handle secretions, food, fluids; observe for drooling.
Assess patient’s ability to blink and speak clearly.
Assess effect of altered appearance on body image.
Risk for Dry Eye related to loss of protective eye closure.
Disturbed Body Image related to facial nerve paralysis.
Administer and teach the patient to administer artificial tears and ophthalmic ointment as prescribed.
Patch eye to keep shut at night, as directed.
Inspect eye for redness or discharge.
Advise the patient to report eye pain immediately.
Encourage the patient to express feelings related to body image disturbance.
Encourage the patient to use mirror as means to obtain feedback about actual versus perceived appearance.
Perform and teach the patient to perform facial massage to alleviate feelings of stiffness and enhance recovery.
Instruct the patient to wear wraparound sunglasses to decrease normal evaporation from the eye from sun and wind, to avoid eye irritants, and to increase environmental humidity.
Instruct the patient in use of ophthalmic drops and ointment, proper methods of lid closure, and patching of the eye.
Demonstrate facial exercises (eg, raise eyebrows, squeeze eyes shut, purse lips) and stress their importance to prevent muscle atrophy.
For further information, refer patient to Bell’s Palsy Research Foundation (www.bellspalsyresearch.com).
Cornea without redness, pain, or discharge.
Verbalizes adjustment to body image disturbance.
 Evidence Base
Evidence Base
Unknown cause, but degenerative or viral origin suspected.
Any of the three trigeminal nerve branches can be affected:
V1: ophthalmic branch; pain involves the eye and forehead.
V2: maxillary branch; pain involves the cheek, upper teeth, upper gums, and nose.
V3: mandibular branch; pain involves the lower jaw, side of tongue, lower teeth, lower gum, extends to ear.
Compression from artery adjacent to the nerve strips myelin from nerve when it pulsates. Loss of myelin acts like an uninsulated wire that “fires” abnormally in response to stimuli.
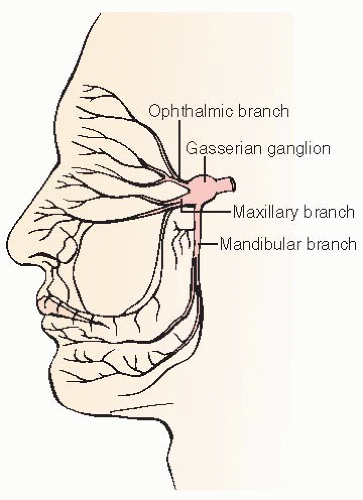 Figure 15-2. The main divisions of the trigeminal nerve are the ophthalmic, maxillary, and mandibular. Sensory root fibers arise in the gasserian ganglion. |
 GERONTOLOGIC ALERT
GERONTOLOGIC ALERT
Sudden, severe episodes of intense facial pain localized to one or more of the branches of the nerve, lasting less than 30 to 60 seconds and ending abruptly.
Pain may occur spontaneously or be precipitated by activation of trigger points, such as touching the face, talking, chewing, yawning, and brushing the teeth, that place pressure on the terminal end of the branch affected.
Pain is always unilateral and does not cross the midline. Bilateral pain is sometimes seen in patients with MS and should result in a high index of suspicion for this condition. MS and hypertension are the two risk factors found in epidemiologic studies.
Some patients experience numbness, particularly around the mouth.
History of characteristic symptoms and pattern; neurologic examination is normal.
Quick response to pharmacologic treatment is important to rule out atypical facial pain, vasomotor or postherpetic neuralgia, and dental pain.
CT scan, MRI, skull x-rays to rule out structural lesions, such as tumor, arteriovenous malformation (AVM), MS, or other disorders.
In contrast to migrainous pain, persons with trigeminal neuralgia rarely suffer attacks during sleep, which is a key point in the history.
Use of carbamazepine is first and most effective medication used to treat the condition. Other drugs, such as imipramine, phenytoin, baclofen, divalproex, gabapentin, or other anti-epileptic drugs (AEDs), may be added or substituted.
Although pain generally responds to pharmacologic intervention, it gradually becomes refractory over time, or patients suffer undesirable adverse effects.
Alcohol, phenol block, or glycerol injection for pain may last several months after injection.
Percutaneous radiofrequency trigeminal gangliolysis directs low-voltage stimulation of nerve by electrode inserted through foramen ovale; sensory function is destroyed with goal to preserve motor function; may cause decreased corneal sensation if V1 affected; paresthesias, jaw weakness, or undesirable, painful numbness (anesthesia dolorosa). Pain may recur as nerve regenerates, necessitating repeat procedure.
Rhizotomy (transection of nerve root at gasserian ganglion) causes complete loss of sensation; other complications include burning, stinging, discomfort in and around eye, herpetic lesions of face, keratitis, and corneal ulceration. Pain may recur as nerve regenerates.
Percutaneous balloon microcompression for selective destruction of nerve fibers that mediate light touch and trigger pain. Relieves pain in ophthalmic branch while sparing corneal sensation.
Microvascular decompression of trigeminal nerve (see “Nursing Management of the Patient Undergoing Intracranial Surgery,” page 492). Most effective form of therapy, with 75% to 80% of patients pain-free without need for long-term medication after procedure. Treatment of choice for younger patients who are low anesthesia risk, do not want facial numbness, and are willing to accept craniotomy.
Gamma knife surgery is less invasive than the percutaneous procedures. It is about as effective as the percutaneous procedures; however, relief may not come for weeks to months and it is a bit more expensive.
Anorexia and weight loss.
Dehydration.
Anxiety, fear.
Depression, social isolation, and suicidal ideations in extreme cases.
Take history of the pain, including duration, severity, and aggravating factors.
Assess nutritional status and hydration.
Assess for anxiety and depression, including problems with sleep, social interaction, coping ability/skills.
Chronic Pain related to physiologic changes of the disorder.
Imbalanced Nutrition: Less Than Body Requirements related to fear of eating.
Powerlessness related to lack of control over painful episodes.
To minimize painful episodes, review with patient potential trigger factors, and develop individualized methods of coping with identified triggers.
Encourage the patient to take medication regularly, including “rescue” medication for breakthrough periods.
Help the patient maintain a method of communication without causing pain from talking.
To maximize nutritional intake, instruct patient to take foods and fluids at room temperature and to chew on the unaffected side.
Have the patient consult with the dietitian for appropriate meal texture and composition.
Encourage small, frequent meals to avoid fatigue and pain.
Advise about use of nutritional supplements, if indicated.
Support patient through treatment trials.
Teach relaxation exercises, such as relaxation breathing, progressive muscle relaxation, and guided imagery, to relieve tension (see page 30).
Encourage participation in support groups (eg, Trigeminal Neuralgia Association) and facilitate a therapeutic relationship with the health care provider.
Educate the surgical patient regarding self-care after denervation procedures.
Instruct the patient to inspect the eye for redness and foreign bodies three to four times per day if corneal sensation is impaired.
Instruct the patient to instill lubricating eye drops every 4 hours if corneal sensation is impaired.
Instruct the patient to avoid drinking hot or very cold liquids.
Instruct the patient to chew on unaffected side to avoid biting tongue, lips, and inside of mouth.
Instruct the patient who wears dentures that jaw muscle will regenerate over time; avoid having dentures refitted, but maintain regular dental checkups because pain will not be felt.
Instruct the patient to report any change in sensation.
Refer patient to the Facial Pain Association for education and support network (www.fpa-support.org) or the National Institute of Neurological Disorders and Stroke (www.ninds.nih.gov).
Verbalizes reduced pain.
Maintains weight.
Verbalizes decreased anxiety and depression.
 Evidence Base
Evidence Base
Cerebrovascular insufficiency can be caused by atherosclerotic plaque or thrombosis, resulting in increased Pco2, decreased Po2, decreased blood viscosity, hyperthermia/hypothermia, increased ICP.
Carotid arteries, vertebral arteries, major intracranial vessels, or microcirculation may be affected.
Cardiac causes of emboli include atrial fibrillation, mitral valve prolapse, infectious endocarditis, and prosthetic heart valve.
Event may be classified as TIA—transient episode of cerebral dysfunction with associated clinical manifestations lasting usually minutes to an hour, possibly up to 24 hours.
Symptoms persisting longer than 24 hours are classified as stroke (also known as brain attack).
Diabetes mellitus (44% risk reduction in hypertensive diabetics with controlled BP).
Hypertension (30% to 40% risk reduction with treatment): goal BP is lower than 140/90; if renal insufficiency or heart failure, less than 130/85; if diabetic, less than 130/80.
Hyperlipidemia (20% to 30% risk reduction with those with known coronary heart disease [CHD] on statin therapy): goal LDL is less than 160 mg/dL if one or no risk factor; less than 130 if two or more risk factors and 10-year CHD risk is less than 20%; less than 100 if two or more risk factors and 10-year CHD risk is greater than 20%.
Coronary artery disease and cardiac disorders, such as congenital heart disease, valvular conditions, endocarditis, atrial fibrillation (68% risk reduction with oral anticoagulation; 21% with aspirin).
History of TIA or stroke.
Rare: Endothelial damage (inflammation or infection, drug-induced, fibromuscular dysplasia, carotid or vertebral artery dissections).
Cigarette smoking (smoking cessation results in a 50% risk reduction in 1 year; return to baseline in 5 years).
Alcohol abuse.
Physical inactivity.
Cocaine use (hemorrhagic stroke).
Increasing age—risk doubles for each decade over age 50.
Gender—men are at greater risk than women.
Heredity—increased risk with family history of stroke.
Ethnic background—blacks and Hispanics at higher risk than whites.
 DRUG ALERT
DRUG ALERT
History of intermittent neurologic deficit, sudden in onset, with maximal deficit within 5 minutes and lasting less than 24 hours.
Carotid system involvement: amaurosis fugax, homonymous hemianopsia, unilateral weakness, unilateral numbness or paresthesias, aphasia, dysarthria.
Vertebrobasilar system involvement: vertigo, homonymous hemianopsia, diplopia, weakness that is bilateral or alternates sides, dysarthria, dysphagia, ataxia, perioral numbness.
Carotid bruit.
History of headaches lasting days before ischemia.
Cerebral angiography, digital subtraction angiography, CT angiography, MRA, Doppler ultrasound—all provide information about carotid arteries, vertebral arteries, and intracranial circulation.
Partial prothrombin time (PTT) and International Normalized Ratio (INR) if anticoagulation is considered. Blood levels are monitored to document therapeutic ranges and determine dosing. PTT is utilized for heparin therapy and INR is utilized for oral warfarin therapy.
MRI stroke protocol: consists of a standard MRI in conjunction with DWI and ADC or PWI scan (see page 476).
Transesophageal echocardiography to rule out emboli from heart.
Platelet aggregation inhibitors, such as aspirin, or clopidogrel to reduce risk of stroke.
Surgical or endovascular intervention to increase blood flow to brain—carotid endarterectomy, extracranial/intracranial anastomosis, transarterial stenting, or angioplasty.
Reduction of other risk factors to prevent stroke, such as control of hypertension, diabetes, and hyperlipidemia, and smoking cessation.
Treatment of arrhythmias.
Treatment of isolated systolic hypertension.
Anticoagulation agents for patients who continue to have symptoms despite antiplatelet therapy (this remains controversial) and those with major source of cardiac emboli.
Complete ischemic stroke (30% of TIAs will progress to stroke within 5 years of initial TIA).
Hemorrhagic conversion of ischemic stroke.
Cerebral edema.
Obtain history of possible TIA; hypertensive and diabetic control; hyperlipidemia; cardiovascular disease or dysrhthmias, such as atrial fibrillation; smoking.
Perform physical examination, including neurologic, cardiac, and circulatory systems; be sure to listen for carotid bruit.
Assess patient for history of headache and, if positive, for duration of headache.
Risk for Ineffective Cerebral Tissue Perfusion related to underlying arteriosclerosis.
Risk for Injury related to surgical procedure.
Readiness for Enhanced Therapeutic Regimen Management regarding therapeutic lifestyle changes.
Teach patient signs and symptoms of TIA and need to notify health care provider immediately. Use the acronym FAST to know what to look for: F, face weakness; A, arm weakness; S, speech difficulties; T, time—immediately seek medical assistance.
Administer or teach self-administration of anticoagulants, antiplatelet agents, antihypertensives, and other medication; also teach about monitoring for adverse effects and therapeutic effect.
Prepare patient for surgical or endovascular intervention as indicated (see page 492).
After surgical procedure, closely monitor vital signs and administer medication as prescribed to avoid hypotension (which can cause cerebral ischemia) or hypertension (which can precipitate cerebral hemorrhage).
Perform frequent neurologic checks, including pupil size, equality, and reaction; handgrip and plantar flexion strength; sensation; mental status; and speech. Notify the health care provider of any deficits immediately.
Observe operative area closely for swelling. Mild swelling is expected, but if hematoma formation is suspected, prepare patient for immediate surgery.
Medicate for pain and avoid agitation or sudden changes in position, which could affect BP.
Elevate head of bed when vital signs are stable.
Following carotid endarterectomy:
Monitor for hoarseness, impaired gag reflex, or difficulty swallowing and facial weakness, which indicate CN injury.
Keep head in neutral position to relieve stress on surgical site; monitor drainage.
Keep tracheostomy tube at bedside and assess for stridor; hematoma formation can cause airway obstruction.
Following extracranial-intracranial anastomosis, avoid pressure over the anastomosis of the superior temporal artery (extracranial) and the middle cerebral artery (intracranial) to prevent rupture or ischemia of the site. If the patient wears glasses, remove the eyeglass arm on the operative side to avoid this possible pressure point.
Following transcranial stenting, administer medications, as directed.
Heparin—bolus given intraprocedure, then possible continuous IV drip postprocedure to maintain PTT within ordered range; monitor PTT every 6 hours.
Clopidogrel before procedure, as ordered. Can be given as a loading dose of 150 mg the evening before procedure, 300-mg loading dose before procedure, 75 mg daily 48 hours before procedure, or 75 mg daily 1 week before procedure. Dosing is physician-dependent.
Daily dosing of 75 mg clopidogrel for 30 to 90 days (specific duration varies), as directed.
Aspirin 81 mg daily, as directed.
Monitor puncture site for bleeding, hematoma, and pulse, as ordered. Apply pressure if bleeding or hematoma is noted and inform physician.
 NURSING ALERT
NURSING ALERT DRUG ALERT
DRUG ALERT
Help the patient begin to formulate a plan for smoking cessation (see page 32).
Teach the patient and family members the basics of nutrition, how to read labels, and how to follow a low-fat diet (particularly one low in saturated fats) (see page 31).
Obtain a referral to a nutritionist for help with weight management and low-fat, low-sodium diet, as indicated.
Encourage daily activity for 30 minutes, if possible. Obtain physical therapy referral for endurance training and monitoring, as indicated.
Monitor INR if warfarin is prescribed and educate patient about risk of bleeding (see page 437).
Encourage the patient receiving long-term oral anticoagulants to comply with follow-up monitoring of INR and to report any signs of bleeding.
Encourage patients receiving antiplatelet agents to report any signs of bleeding.
Encourage the use of electric razors and toothbrushes to prevent bleeding.
Reinforce with patient and family the importance of accessing the medical system, by calling 911, when symptoms first occur.
Refer the patient to the American Stroke Association (www.strokeassociation.org) or the National Institute of Neurologic Disorders and Stroke (www.ninds.nih.gov) for additional information and support.
Alert without neurologic deficits.
Respirations unlabored, vital signs stable, no swelling of neck; reports relief of pain.
Expresses readiness to quit smoking and adhere to a low-fat diet.
 Evidence Base
Evidence Basehypertension. Stroke is the leading cause of long-term disability and the third leading cause of death in the United States, with an annual incidence of 700,000.
Partial or complete occlusion of a cerebral blood flow to an area of the brain due to:
Thrombus (most common)—due to arteriosclerotic plaque in a cerebral artery, usually at bifurcation of larger arteries; occurs over several days.
Embolus—a moving clot of cardiac origin (frequently due to atrial fibrillation) or from a carotid artery that travels quickly to the brain and lodges in a small artery; occurs suddenly with immediate maximum deficits.
Area of brain affected is related to the vascular territory that was occluded. Subtle decrease in blood flow may allow brain cells to maintain minimal function, but as blood flow decreases, focal areas of ischemia occur, followed by infarction to the vascular territory. See Figure 15-3 for cerebral circulation.
An area of injury includes edema, tissue breakdown, and small arterial vessel damage. The small arterial vessel damage poses a risk of hemorrhage. The larger the area of infarction, the greater the risk of hemorrhagic conversion.
Ischemic strokes are not activity-dependent; may occur at rest.
 NURSING ALERT
NURSING ALERT
Leakage of blood from a blood vessel and hemorrhage into brain tissue, causing edema, compression of brain tissue, and spasm of adjacent blood vessels.
May occur outside the dura (extradural), beneath the dura mater (subdural), in the SAS, or within the brain substance (intracerebral).
Causal mechanisms include:
Increased pressure due to hypertension.
Head trauma causing dissection or rupture of vessel.
Deterioration of vessel wall from chronic hypertension, diabetes mellitus, or cocaine use.
Congenital weakening of blood vessel wall with aneurysm or AVM.
The intracranial hemorrhage becomes a space-occupying lesion within the skull and compromises brain function.
The mass effect causes pressure on brain tissue.
The hemorrhage irritates local brain tissue, leading to surrounding focal edema.
SAH or hemorrhage into a ventricle can block normal CSF flow, leading to hydrocephalus.
Hemorrhage commonly occurs suddenly while a person is active.
Clinical manifestations vary depending on the vessel affected and the cerebral territories it perfuses. Symptoms are usually multifocal. Headache may be a sign of impending cerebral hemorrhage or infarction; however, it is not always present.
Common clinical manifestations related to vascular territory (see Table 15-5):
Numbness (paresthesia), weakness (paresis), or loss of motor ability (plegia) on one side of the body.
Difficulty in swallowing (dysphagia).
Aphasia (nonfluent, fluent, global).
Visual difficulties of inattention or neglect (lack of acknowledgment of one side of the sensory field), loss of half of a visual field (hemianopsia), double vision, photophobia.
Altered cognitive abilities and psychological affect.
Self-care deficits.
Carotid ultrasound—to detect carotid stenosis in ischemic stroke.
CT scan—to determine cause and location of stroke and type of stroke, ischemic versus hemorrhagic.
MRA or CT angiogram—noninvasive evaluation of cerebrovascular structures in ischemic stroke.
Cerebral angiography—invasive evaluation of cerebrovasculature to determine the extent of cerebrovascular injury/insufficiency and to evaluate for structural abnormalities.
PET, MRI with DWI—to localize ischemic damage in ischemic stroke.
CT perfusion/MR perfusion—provides evaluation of cerebral blood flow, cerebral blood volume, and mean transient time to determine the extent of ischemic changes.
TCD—noninvasive method used to evaluate cerebral perfusion. Useful in the bedside evaluation and to provide a means for ongoing monitoring of cerebral blood flow to document changes and trends.
Table 15-5 Stroke Deficits Related to Vascular Territory | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Support of vital functions—maintain airway, breathing, oxygenation, circulation.
Maintain pulse oximetry greater than 92%. Utilize oxygen therapy, as needed.
Arrhythmias are common. Telemetry may be indicated to monitor. Atrial fibrillation is the most common arrhythmia in stroke population.
Astute neurologic assessment utilizing the National Institute of Health Stroke Scale (www.ninds.nih.gov/doctors/nih/_stroke_scale_booklet.pdf) and management of increased ICP (see page 486).
IV fluids (normal saline) at maintenance until able to tolerate oral diet. Colloids may be used for reperfusion and hemodilution.
Maintain BP within prescribed parameters. Limit BP fluctuations.
Management of systemic hypertension with labetalol, nicardipine, or alternative IV antihypertensive agents to keep systolic BP (SBP) less than 200 mm Hg. Goal is to promote adequate cerebral perfusion to prevent further ischemia. Avoid lowering BP greater than 25% within the first 24 hours. Hemorrhagic conversion/transformation can occur and is associated with degree of necrotic tissue and vascular injury. Hyperglycemia and BP fluctuations or rapid increases in BP increase the risk of hemorrhagic conversion.
Management of hypotension—SBP goal > 100. Vasopressor agents to maintain SBP within prescribed range. Sustained hypotensive episodes are associated with poor outcome.
Pretreatment with tissue plasminogen activator (tPA)— unable to receive tPA if SBP > 185 mm Hg or diastolic BP > 110 mm Hg.
Post-tPA—maintain SBP < 180 mm Hg or diastolic BP < 105 mm Hg for first 24 hours post tPA.
Thrombolytic therapy (see Box 15-3, page 502).
Recombinant tPA administered IV is the only Food and Drug Administration (FDA)-approved medical treatment for acute ischemic stroke. Dosing: IV 0.9 mg/kg within 3 hours of onset of symptoms.
Transarterial tPA within 6 hours of onset of symptoms. Benefits have been demonstrated in acute ischemic stroke related to occlusion of middle cerebral artery. Advantages include:
Higher concentration delivered to clot.
Can be performed in conjunction with mechanical disruption of clot.
Provides precise imaging of pathology and evaluation of collateral circulation.
Defines extent of injury and recanalization.
Disadvantages include:
Risk of hemorrhage related to catheter manipulation.
Dislodgement of clot.
Delay in thrombolytic therapy due to access delays.
Limited facility-based accessibility.
Additional treatment options (transarterial or endovascular):
Abciximab—antiplatelet agent delivered intra-arterially (IA).
Verapamil—potent vasodilator injected into intracranial vessel to treat acute spasm.
Clot retrieval or manipulation of clot.
Angioplasty and stenting have been utilized in acute dissection, though data are limited.
Maintain normal glucose levels because hypoglycemia and hyperglycemia are associated with a poor outcome. Sliding scale insulin or insulin drip should be given to maintain normoglycemia.
Maintain normothermia because hyperthermia is associated with a poor neurologic outcome.
Deep vein thrombosis prophylaxis—sequential stockings and low-dose heparin or low-molecular-weight heparin (LMWH) should be utilized. Data does not suggest any increased risk of hemorrhage in this population.
Focus on early rehabilitation.
Onset of symptoms
Within 3 hours IV tPA.
Within 6 hours IA tPA.
Ischemic stroke with measurable deficits using the NIH Stroke Scale (see page 501).
No hemorrhage noted on CT scan of brain.
Clearly defined time of symptom onset.
Head CT demonstrates early signs of infarction or hemorrhage.
Uncontrolled hypertensive nonresponsive to IV or oral agent (systolic > 185 or diastolic > 110 mm Hg).
Glucose level > 400 mg/dL.
Coagulopathy.
Heparin within past 48 hours.
Patient on warfarin.
Elevated PTT or prothrombin time.
Platelet count < 100,000.
History of previous intracranial hemorrhage, head trauma, or stroke within past 3 months.
GI or genitourinary tract hemorrhage in the past 3 weeks.
History of major surgery in the past 2 weeks.
Support of vital functions—maintain airway, breathing, oxygenation, circulation.
Maintain pulse oximetry greater than 92%. Utilize oxygen therapy, as needed.
Arrhythmias are common. Telemetry may be indicated to monitor.
IV fluids (normal saline) at maintenance until able to tolerate oral diet.
Neurologic assessment—Facility-based neurological assessment tool should be used. Notify health care provider of clinical deterioration. Deterioration can be related to rebleed or development of cerebral edema.
Reversal of coagulopathies:
Obtain patient’s medication history. Medications to be concerned about include warfarin, aspirin, clopidogrel, ticlopidine, and dipyridamole/aspirin.
Obtain INR and platelet function analyzer (PFA-100), as ordered. Elevated INR is associated with warfarin use. PFA epinephrine greater than 164 denotes platelet dysfunction related to aspirin use and PFA adenosine diphosphate greater than 116 denotes nonaspirin platelet dysfunction and has been used to determine the effectiveness of clopidogrel or ticlopidine or the presence of von Willebrand’s disease.
As ordered, treat with platelets, cryoprecipitate, or vitamin K to reverse coagulopathy. Prothrombin complex concentrate may be used for rapid reversal.
Management of BP within prescribed parameters—maintain SBP < 160 to reduce vessel wall stressors. Goal is to prevent rebleeding while promoting adequate cerebral perfusion. Labetalol, hydralazine, nicardipine, or alternative IV antihypertensive agents may be used.
Neurosurgical consultation for possible evacuation of intracerebral hemorrhage may be explored.
Prophylactic treatment of seizures with phenytoin. Seizures commonly occur within the first 24 hours of intracerebral hemorrhage.
Maintain normal glucose level because hypoglycemia/hyperglycemia are associated with a poor outcome. Sliding scale insulin or insulin drip should be given to maintain normoglycemia.
Maintain normothermia because hyperthermia is associated with a poor neurologic outcome. Current data does not support the use of therapeutic, or induced, hypothermia in patients with ischemic stroke; however, research is ongoing to determine potential applications.
Deep vein thrombosis prophylaxis—sequential stockings and low-dose heparin or LMWH should be utilized. Data do not suggest any increased risk of hemorrhage in this population.
Focus on early rehabilitation.
Anticoagulation may be instituted after hemorrhage is ruled out. However, anticoagulation remains controversial because current literature suggests acute use of heparin increases the risk of hemorrhagic conversion and use of warfarin has
demonstrated no significant benefit unless the patient is in atrial fibrillation. Anticoagulation is contraindicated for the first 24 hours in patients who received tPA.
Aspirin is the only antiplatelet agent that has been evaluated in acute ischemic stroke. Aspirin is recommended within 24 to 48 hours poststroke. (In tPA patients, aspirin should not be given until 24 hours post-tPA.) Alternative antiplatelet agents include ticlopidine, dipyridamole/aspirin 200/25, and clopidogrel.
Antispasmodic agents can be used for spastic paralysis.
Early initiation of a rehabilitation program, including physical therapy, occupational therapy, and speech therapy, and counseling, as needed.
Depression is common; therefore, early initiation of antidepressants can be beneficial, such as selective serotonin reuptake inhibitors.
Aspiration pneumonia.
Dysphagia in 25% to 50% of patients after stroke.
Spasticity, contractures.
Deep vein thrombosis, pulmonary embolism.
Brain stem herniation.
Poststroke depression.
Maintain neurologic flow sheet (National Institutes of Health (NIH) Stroke Scale for ischemic stroke and facility-based neurological assessment tool for hemorrhagic stroke).
Assess for voluntary or involuntary movements, tone of muscles, presence of deep tendon reflexes (reflex return signals end of flaccid period and return of muscle tone).
Also assess mental status, CN function, sensation/proprioception.
Monitor bowel and bladder function/control.
Monitor effectiveness of anticoagulation therapy.
Frequently assess level of function and psychosocial response to condition.
Assess for skin breakdown, contractures, and other complications of immobility.
 DRUG ALERT
DRUG ALERT
Risk for Injury related to neurologic deficits.
Impaired Physical Mobility related to motor deficits.
Impaired Environmental Interpretation Syndrome related to brain injury.
Impaired Verbal Communication related to brain injury.
Bathing, Dressing, Toileting Self-Care Deficit related to hemiparesis/paralysis.
Imbalanced Nutrition: Less Than Body Requirements related to impaired self-feeding, chewing, swallowing.
Impaired Urinary Elimination related to motor/sensory deficits.
Disabled Family Coping related to catastrophic illness, cognitive and behavioral sequelae of stroke, and caregiving burden.
 NURSING ALERT
NURSING ALERT
Maintain bed rest during acute phase (24 to 48 hours after onset of stroke) with head of bed slightly elevated and side rails in place.
Administer oxygen, as ordered, during acute phase to maximize cerebral oxygenation.
Frequently assess respiratory status, vital signs, heart rate and rhythm, and urine output to maintain and support vital functions.
When patient becomes more alert after acute phase, maintain frequent vigilance and interactions aimed at orienting, assessing, and meeting the needs of the patient.
Try to allay confusion and agitation with calm reassurance and presence.
Assess patient for risk for fall status.
Maintain functional position of all extremities.
Apply a trochanter roll from the crest of the ilium to the midthigh to prevent external rotation of the hip.
Place a pillow in the axilla of the affected side when there is limited external rotation to keep arm away from chest and prevent adduction of the affected shoulder.
Place the affected upper extremity slightly flexed on pillow supports with each joint positioned higher than the preceding one to prevent edema and resultant fibrosis; alternate elbow extension.
Place the hand in slight supination with fingers slightly flexion.
Avoid excessive pressure on ball of foot after spasticity develops.
Do not allow top bedding to pull affected foot into plantar flexion; may use tennis shoes in bed.
Place the patient in a prone position for 15 to 30 minutes daily, and avoid sitting up in chair for long periods to prevent knee and hip flexion contractures.
Encourage neutral positioning of affected limbs to promote relaxation and to limit abnormal increases in muscular tone to enhance functional recovery (reflex-inhibiting positioning).
“Forced use” is an experimental treatment designed to overcome nonuse of the hemiparetic upper extremity in regaining functional use of the affected arm with selected chronic hemiparetic patients.
“Constraint-induced movement therapy” restricts the contralateral upper extremity in effort to force use of the affected arm.
Apply splints and braces, as indicated, to support flaccid extremities or on spastic extremities to decrease stretch stimulation and reduce spasticity.
Volar splint to support functional position of wrist.
Sling to prevent shoulder subluxation of flaccid arm.
High-top sneaker for ankle and foot support.
Exercise the affected extremities passively through ROM four to five times daily to maintain joint mobility and enhance circulation; encourage active ROM exercise as able (see page 174).
Teach patient to use unaffected extremity to move affected one.
Assist with ambulation, as needed, with help of physical therapy, as indicated.
Check for orthostatic hypotension when dangling and standing.
Gradually position the patient from a reclining position to head elevated, and dangle legs at the bedside before transferring out of bed or ambulating; assess sitting balance in bed.
Assess the patient for excessive exertion.
Have patient wear walking shoes or tennis shoes.
Assess standing balance and have patient practice standing.
Help patient begin ambulating as soon as standing balance is achieved; ensure safety with a patient waist belt.
Provide rest periods as patient will tire easily.
Be aware of the patient’s cognitive alterations and adjust interaction and environment accordingly.
Participate in cognitive retraining program—reality orientation, visual imagery, cueing procedures—as outlined by rehabilitation nurse or therapist.
In patients with increased awareness, use pictures of family members, clock, calendar; post schedule of daily activities where patient can see it.
Focus on patient’s strengths and give positive feedback.
Be aware that depression is common and therapy should include psychotherapy and early initiation of antidepressants.
Speak slowly, using visual cues and gestures; be consistent and repeat as necessary.
Speak directly to the patient while facing him or her.
Give plenty of time for response and reinforce attempts as well as correct responses.
Minimize distractions.
Use alternative methods of communication other than verbal, such as written words, gestures, or pictures.
Teach the patient to use nonaffected side for activities of daily living (ADL) but not to neglect affected side.
Adjust the environment (eg, call light, tray) to side of awareness if spatial neglect or visual field cuts are present; approach patient from uninvolved side.
Teach the patient to scan environment if visual deficits are present.
Encourage the family to provide clothing a size larger than patient wears, with front closures, Velcro, and stretch fabric; teach the patient to dress while sitting to maintain balance.
Make sure personal care items, urinal, and commode are nearby and that patient obtains assistance with transfers and other activities, as needed.
Be aware that ADL require anticipatory (automatic coordination of multiple muscle groups in anticipation of a specific movement) and reactive (adjustment of posture to stimuli) postural adjustments.
Be aware that patients usually have clear goals in relation to functional abilities, against which all success and forward progress will be measured; help them set realistic short- and long-term goals.
Perform a bedside swallow screen. Follow institutional protocol. If any coughing or difficulties are noted, contact speech therapy for official consult.
Initiate referral for a speech therapist for individuals with compromised LOC, dyspraxic speech, or speech difficulties to evaluate swallowing function at bedside or radiographically to demonstrate safe and functional swallowing mechanisms before initiation of oral diet.
Help the patient relearn swallowing sequence using compensatory techniques.
Place ice on tongue and encourage sucking.
Progress to ice pops and soft foods.
Make sure mechanical soft or pureed diet is provided, based on ability to chew.
Encourage small, frequent meals and allow plenty of time to chew and swallow. Dietary consults can be helpful for selection of food preferences.
Remind the patient to chew on unaffected side.
Encourage the patient to drink small sips from a straw with chin tucked to the chest, strengthening effort to swallow while chin is tucked down.
Inspect mouth for food collection and pocketing before entry of each new bolus of food.
Inspect oral mucosa for injury from biting tongue or cheek.
Encourage frequent oral hygiene.
Teach the family how to assist the patient with meals to facilitate chewing and swallowing.
Reduce environmental distractions to improve patient concentration.
Provide oral care before eating to improve aesthetics and afterward to remove food debris.
Position the patient so he or she is sitting with 90 degrees of flexion at the hips and 45 degrees of flexion at the neck. Use pillows to achieve correct position.
Maintain position for 30 to 45 minutes after meals to prevent regurgitation and aspiration.
Insert indwelling bladder catheterization during acute stage for accurate fluid management; remove as soon as status stabilizes.
Establish regular voiding schedule—every 2 to 3 hours, correlated with fluid intake—when bladder tone returns. If the patient is unable to void, intermittent catheterization can be used to empty bladder and prevent overstretching of bladder. The bladder scan device is useful in monitoring bladder capacity and identifying individuals at risk.
Assist with standing or sitting to void (especially males).
See page 181 for bladder retraining program details.
Consult a social worker for assistance with patient/family support, to obtain copy of durable power of attorney, address guardianship, and long-term care decisions, as needed.
Encourage the family to maintain outside interests.
Teach stress management techniques, such as relaxation exercises, use of community and faith-based support networks.
Encourage participation in support group for family respite program for caregivers or other available resources in area.
Involve as many family and friends in care as possible.
Provide information about stroke and expected outcome.
Teach the family that stroke survivors may show depression in the first 3 months of recovery.
Hemiplegic complications resulting from stroke commonly include “frozen” shoulder; adduction and internal rotation of arm with flexion of elbow, wrist, and fingers; external rotation of the hip with flexion of the knee and plantar flexion of the ankle.
Perform ROM exercises and instruct the patient and family on these as well as on proper positioning.
Reinforce that these muscle and ligament deformities resulting from stroke can be prevented with daily stretching and strengthening exercises.
Depression after stroke is a major problem because it can increase morbidity. Monitor for signs of depression, such as difficulty sleeping, frequent crying, anorexia, feelings of guilt or sadness. Notify the health care provider for possible medication therapy.
Continue to support family who may be caring for a hemiplegic or aphasic person at home or in long-term care for a long time. Six months after stroke, around 9% to 21% of survivors are severely disabled, fully dependent, and live in institutions.
Teach the patient and family to adapt home environment for safety and ease of use.
Instruct the patient of the need for rest periods throughout day.
Reassure the family that it is common for poststroke patients to experience emotional lability and depression; treatment can be given.
Encourage consistency in the environment without distraction.
Assist the family to obtain self-help aids for the patient.
Instruct the family in management of aphasia (see Box 15-4, page 506).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



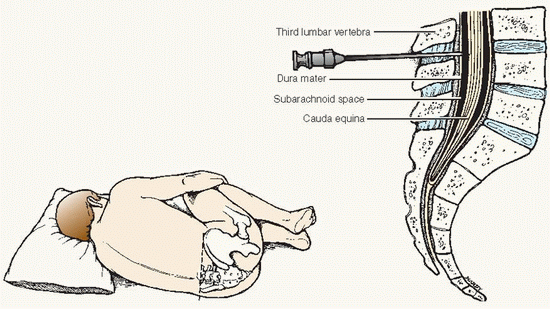
 Evidence Base American Association of Neuroscience Nurses. (2011). Care of the patient undergoing intracranial pressure monitoring/external ventricular drainage or lumbar drainage. Glenview, IL: Author.
Evidence Base American Association of Neuroscience Nurses. (2011). Care of the patient undergoing intracranial pressure monitoring/external ventricular drainage or lumbar drainage. Glenview, IL: Author.
 Key Decision Point Check ICP waveforms before nursing interventions such as turning or bathing the patient. The presence of an elevated P-2 wave indicates reduced cerebral compliance, meaning that the normal compensatory responses may not be effective in decreasing ICP. Thus, extra precautions and preventive actions should be taken prior to exposing the patient to additional stimuli. Nursing interventions may need to be postponed until cerebral compliance increases. Alert the health care provider if ICP greater than 20 mm Hg and prepare for therapeutic intervention.
Key Decision Point Check ICP waveforms before nursing interventions such as turning or bathing the patient. The presence of an elevated P-2 wave indicates reduced cerebral compliance, meaning that the normal compensatory responses may not be effective in decreasing ICP. Thus, extra precautions and preventive actions should be taken prior to exposing the patient to additional stimuli. Nursing interventions may need to be postponed until cerebral compliance increases. Alert the health care provider if ICP greater than 20 mm Hg and prepare for therapeutic intervention.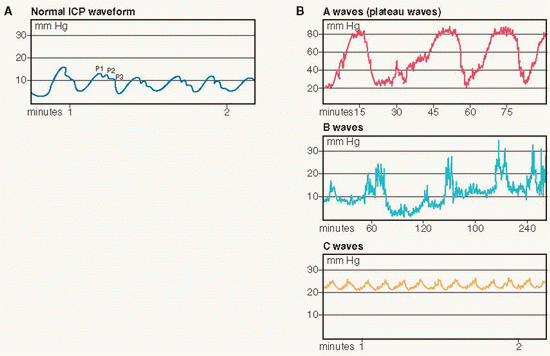 (A) Normal waveform; (B) A waves (plateau waves), B waves, and C waves.
(A) Normal waveform; (B) A waves (plateau waves), B waves, and C waves. Evidence Base American Association of Neuroscience Nurses. (2011). Care of the patient undergoing intracranial pressure monitoring/external ventricular drainage or lumbar drainage. Glenview, IL: Author.
Evidence Base American Association of Neuroscience Nurses. (2011). Care of the patient undergoing intracranial pressure monitoring/external ventricular drainage or lumbar drainage. Glenview, IL: Author.