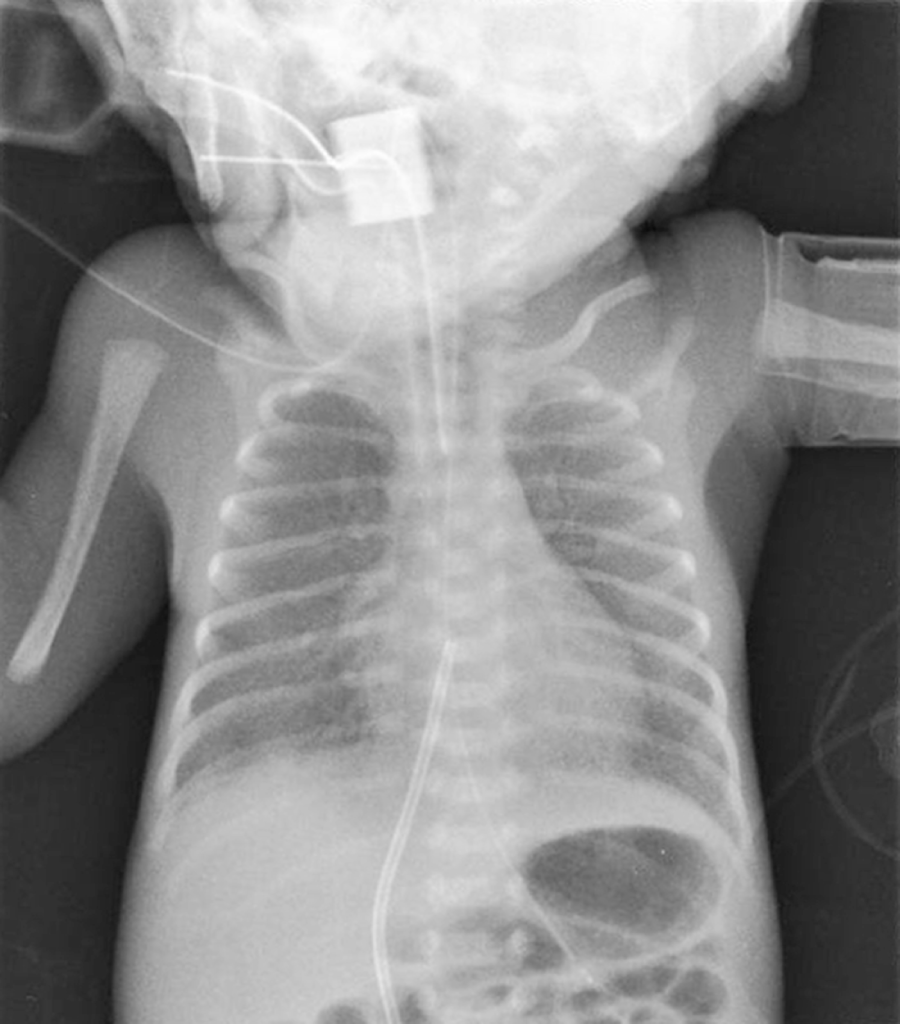
Susanne Simmons This chapter will focus on the respiratory management of a preterm infant with RDS. Although the scenario is based on a premature infant, some of the principles of care outlined in the chapter are also relevant to the term neonate and infant. The neonatal mortality rate has declined significantly since 2000 (ONS 2019) with the demand for neonatal care increasing each year (RCPCH 2020). Approximately one in seven infants born each year will require admission to a neonatal unit, with preterm infants requiring specialist neonatal intensive care (BLISS 2021). Activity of daily living: breathing. The lungs in utero are filled with foetal lung fluid and the volume of this and amniotic fluid is crucial to normal lung development (Randall 2005). In addition, foetal breathing movements, seen in utero from as early as 12 weeks, are also thought to support the development of the diaphragm and chest wall muscles. Lung development is characterised by stages (Moore, Persaud and Torchia 2019), which overlap and do not occur uniformly within each lung (see Table 19.1). The lungs need to be developed sufficiently to enable the alveoli to be involved in gaseous exchange and this is usually achieved by 32–34 weeks’ gestation. At 27 weeks’ gestation, Arthur’s lungs will be immature in both structure and the amount of surfactant produced. Table 19.1 Overview of the stages of lung development. Respiratory distress syndrome is a pulmonary disorder caused by a lack of surfactant and is predominantly associated with prematurity. Surfactant is a complex mixture of proteins, lipids, and phospholipids and spreads across the liquid lined surface of the alveoli (Crawford & Davies 2020). It reduces surface tension and prevents the collapse (atelectasis) of the alveoli on expiration, therefore maintaining a positive pressure in the airways (functional residual capacity). The liquid lined surface of the alveoli facilitates normal gaseous exchange. It is known that during a normal vaginal delivery the release of adrenaline promotes the switch of lung cells from producing foetal lung fluid to absorbing it. In addition, the pressure applied to the infant’s ribcage during the delivery ‘squeezes’ the lung fluid out through the mouth and nose. As the infant takes its first breath a high intrathoracic pressure is generated which pushes the lung fluid into the pulmonary vascular and lymphatic system (Moore et al. 2019). As Arthur was delivered by an emergency LSCS, this process did not occur. Antenatal corticosteroids, given in time to have an effect, cross the placenta and appear to accelerate lung development (Sweet et al. 2019). However, antenatal steroids were not given prior to delivery. Arthur is therefore challenged at delivery in terms of his respiratory function. Neonates are predominantly nose breathers for the first few months of life. Arthur’s airways are extremely narrow and his large tongue in relation to his small jaw can allow the tongue to fall back and obstruct his upper airway. In addition, the gag reflex is underdeveloped, and the large occiput of the neonate’s head can push the chin downwards onto the chest when lying supine and cause airway obstruction. Due to surfactant deficiency his alveoli are collapsed (atelectasis) and a chest X-ray (Figure 19.1) reveals the lung fields have a ground glass appearance, with air bronchograms (the air-filled left and right main bronchi standing out black against the collapsed white lung fields). FIGURE 19.1 Chest X-ray. A normal respiratory rate for the neonate is below 60 breaths per minute. Without respiratory support, Arthur will demonstrate signs of respiratory distress due to surfactant deficiency. These signs include nasal flaring, subcostal (underneath ribcage) recession, intercostal (in-between ribs) recession, sternal recession, tracheal tug, and an expiratory grunt. Grunting occurs when an infant breathes out against a partially closed glottis in order to prevent alveoli collapse and preserve lung volume. Due to his compliant ribcage and small airways, Arthur also uses accessory abdominal muscles to ensure effective air entry. Therefore, a diaphragmatic breathing pattern is observed. Atelectasis results in ineffective gaseous exchange which means that Arthur would be hypoxic and hypercarbic (high carbon dioxide). Oxygen saturation monitoring and blood gas analysis is important in assessing respiratory function and metabolic parameters (Lynch 2009). Arthur’s initial arterial blood gas on admission revealed a respiratory acidosis: pH 7.19, PaCO2 8.9, PaO2 6.0, base excess – 2, standard bicarbonate 20.
CHAPTER 19
Neonatal Respiratory Distress Syndrome
ANSWERS TO QUESTIONS
Question 1. Assessment: How would you assess Arthur’s respiratory needs?
Pseudo glandular stage (6–16 weeks)
The trachea, bronchi, and bronchioles are formed. Connective tissue, muscle, and blood vessels start to develop.
Canalicular stage (16–25 weeks)
The bronchioles continue to branch, and the vascular network develops alongside the airways. Type 2 cells begin to secrete small amounts of surfactant. The cells lining the alveolar are cuboidal, epithelial cells and are thick.
Terminal saccular stage (24 weeks to birth)
The alveoli develop and multiply. The alveolar epithelial lining thins (squamous epithelium) which allows closer contact with the capillaries. There is a surge of surfactant produced at 32–34 weeks’ gestation.
Alveolar stage (32 weeks to childhood)
This stage continues after birth and into childhood. The alveoli develop further and mature. There are approximately 50 million alveoli present in the full-term infant and this reaches adult values of 300 million during childhood.
Respiratory assessment of Arthur: airway and breathing
Airway
Breathing
Question 2. Planning: What respiratory support will Arthur require?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


