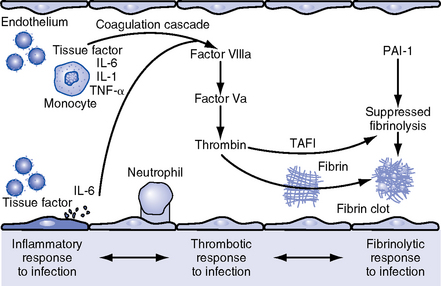
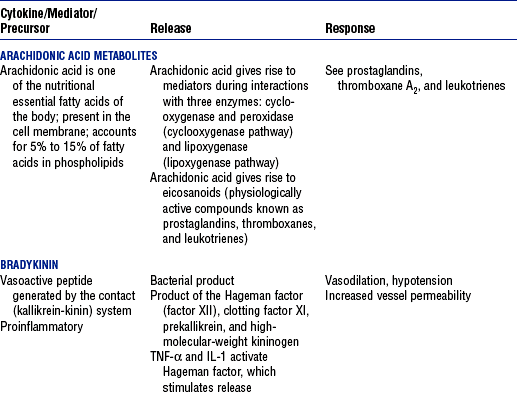
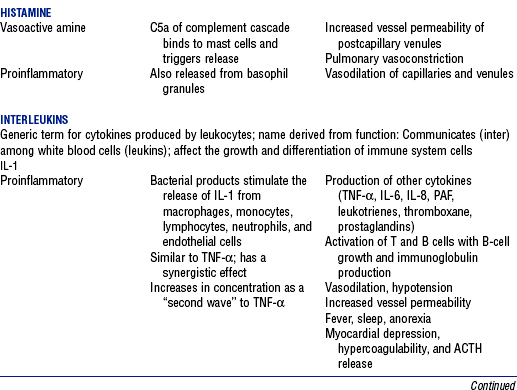
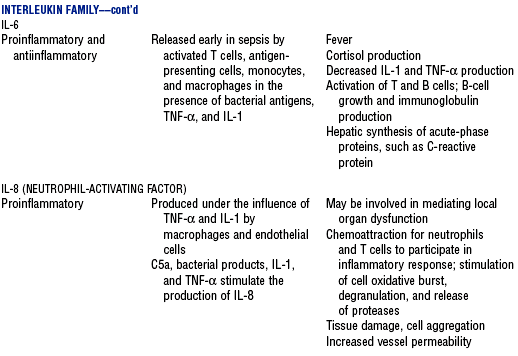
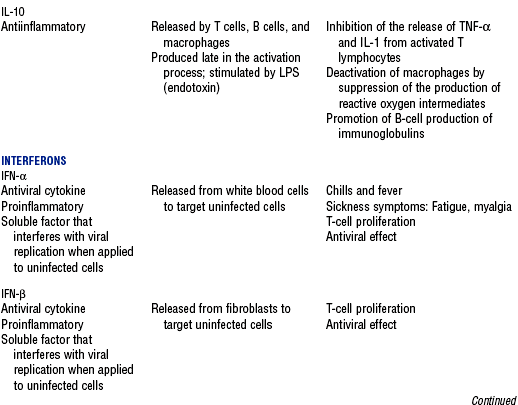
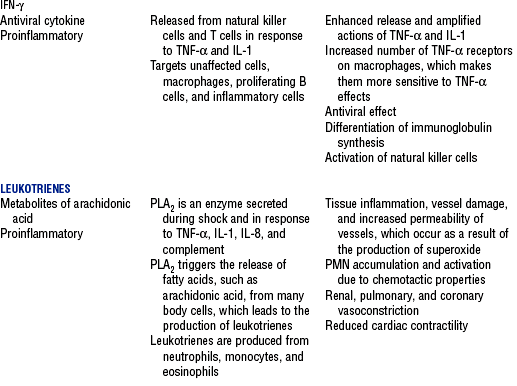
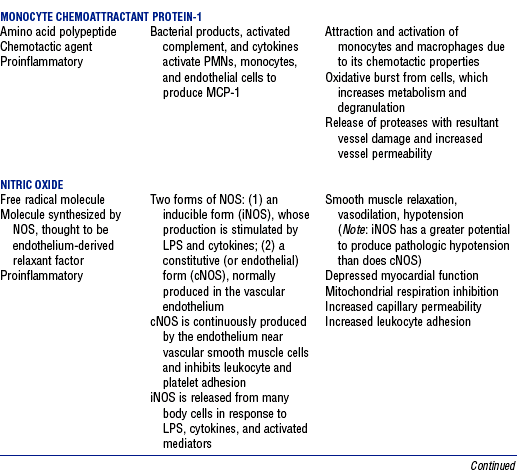
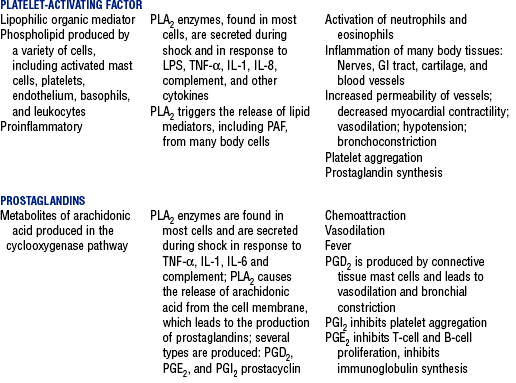
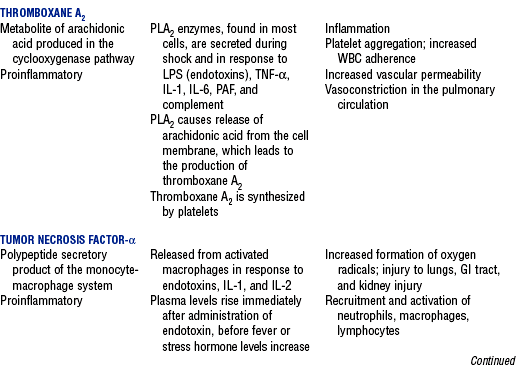
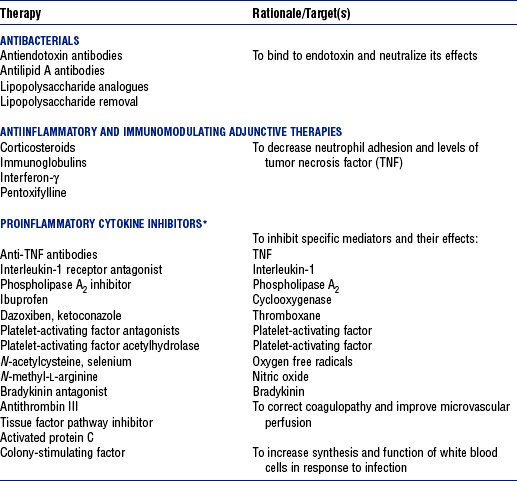
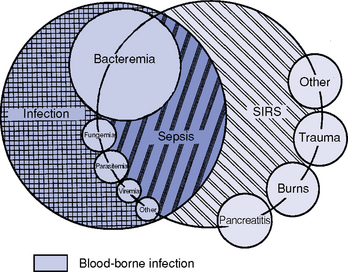
CHAPTER 9 a. Systemic inflammatory response syndrome (SIRS): Systemic inflammatory response to a variety of severe clinical insults (such as pancreatitis, ischemia or reperfusion, multiple trauma and tissue injury, hemorrhagic shock, and immune-mediated organ injury) in the absence of infection (Figure 9-1). Response is manifested by two or more of the following conditions: FIGURE 9-1 Interrelationship between systemic inflammatory response syndrome (SIRS), sepsis, and infection. (From Bone RC, Balk RA, Cerra FB, et al: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis, Chest 101:1645, 1992.) i. Temperature above 100.4° F (38° C) or below 96.8° F (36° C) ii. Heart rate above 90 beats/min iii. Respiratory rate above 20 breaths/min or arterial partial pressure of carbon dioxide (Paco2) below 32 mm Hg iv. White blood cell (WBC) count above 12,000/mm3 or below 4000/mm3, or more than 10% immature (band) forms b. Infection: Microbial phenomenon characterized by an inflammatory response to the presence of microorganisms or the invasion of normally sterile host tissue by organisms c. Bacteremia: Presence of viable bacteria in the blood d. Sepsis: Bacterial infection of the blood i. In 2002, the PIRO model was developed as a tool to diagnose and track the progression of sepsis (a) P: Predisposition for individual patients to respond to infection in different ways (c) R: Response to inflammation ii. Sepsis may be a complication following burns, surgery, or illness iii. Sepsis is associated with a generalized inflammatory response that often leads to abnormal clotting and bleeding in the presence of infection iv. This condition is manifested by the presence of two or more of the four conditions that define SIRS (see earlier) e. Severe sepsis: Sepsis associated with organ dysfunction. Associated signs and symptoms include chills, tachypnea, unexplained alterations in mental status, tachycardia, altered WBC count, decreased platelet count, elevated numbers of immature neutrophils, decreased skin perfusion, decreased urine output, skin mottling, poor capillary refill, hypoglycemia, and petechiae. f. Septic shock: Sepsis-induced state with hypotension despite adequate volume resuscitation along with perfusion abnormalities that may include, but are not limited to, lactic acidosis, oliguria, and acute alterations in mental status. Frequently, patients have cardiovascular system failure as evidenced by hypotension and reduced perfusion to vital organs. Patients receiving inotropic or vasopressor agents may not be hypotensive at the time that perfusion abnormalities are measured. g. Multiple organ dysfunction syndrome (MODS): Presence of progressive physiologic dysfunction in two or more organ systems after an acute threat to systemic homeostasis a. Sepsis is the tenth leading cause of death in the United States i. Sepsis is the leading cause of death in intensive care units (ICUs) ii. Sepsis develops in more than 750,000 people annually, with 2000 new cases per day in the United States iii. Approximately 40% of patients with sepsis develop septic shock b. Mortality rates in the United States: Approximately 215,000 people die each year of either septic shock or bacteremia i. Sepsis due to gram-negative organisms carries a mortality rate of 20% to 50% ii. Severe sepsis carries a mortality rate of 28% to 50% or higher and is present in 6.3% of all patients admitted to the ICU c. Health care costs of severe sepsis exceed $17 billion annually 3. Influences of gender on the response to sepsis: Gender-based differences exist in the response to infection and sepsis a. Females: Estrogen may enhance immune function to the extent of inducing autoimmune disease i. Estrogen provides a protective effect in the presence of sepsis ii. Monocytes produce more interleukin (IL)-1, cause chemotaxis and phagocytosis b. Males: Testosterone suppresses immune function, placing males at risk for worse outcomes; androgens depress the immune response c. Once sepsis or septic shock develops, there is no difference in the mortality rate between males and females a. Many experts agree that, prior to the development of SIRS, a physiologic insult occurs. The insult may take the form of an infection, traumatic injury, surgical incision, burn injury, or pancreatitis. The initial physiologic response to the insult is the development of a proinflammatory state characterized by the expression of multiple mediators in an effort to limit damage from the insult (Figure 9-2). b. Gram-positive bacteria are responsible for approximately 50% of infections resulting in sepsis; gram-negative bacteria account for approximately 25%; 15% of the infections are due to a mix of gram-positive and gram-negative organisms; fungal pathogens account for 5% to 10% of the infections c. When phagocytic cells destroy bacteria, a cascade of events follows. Sequence of events varies depending on whether gram-negative or gram-positive organisms are involved. d. All gram-negative bacteria have a common group of molecules in the outer membrane, referred to as lipopolysaccharide (LPS) or endotoxin i. LPS is composed of lipid A and a polysaccharide core linked to an “O-polysaccharide” side chain of repeating sugars (a) Lipid A and the polysaccharide core are identical or nearly identical in most gram-negative bacteria (b) O-polysaccharide varies for each specific gram-negative organism ii. LPS (in particular, lipid A) interacts with the body’s immune system to produce a septic state iii. LPS binds to a circulating LPS-binding protein to form a complex in the blood (a) Complex can then bind to a macrophage membrane receptor (CD14), which presents LPS to a signal-transducing receptor in the cell membrane (b) Binding triggers the production of mediators known as cytokines (soluble molecules released from cells of the immune system whose function is to signal to other cells) (Table 9-1); the proinflammatory cytokine activity attracts further macrophages and monocytes, which results in a repetitive cycle e. Components of the gram-positive bacteria cell wall, such as lipoteichoic acid or peptidoglycan, bind to receptors to stimulate cytokine release; bacterial components stimulate the coagulation and complement cascades f. Regardless of the inciting pathogen, the cytokines involved in the massive inflammatory reaction known as SIRS may lead to multiple organ dysfunction and are similar to those released in septic shock g. Consequences of cytokine production i. Systemic vasodilation with decreased afterload and hypotension ii. Increased capillary permeability with decreased preload, third-spacing, and interstitial edema iv. Decreased tissue oxygen extraction v. Platelet aggregation, fibrin deposits, and activation of a clotting cascade, leading to microcirculatory coagulation, maldistribution of blood flow, and tissue hypoxia i. Proinflammatory cytokine production and an inflammatory process are normally strongly repressed by the antiinflammatory compensatory response mechanisms ii. SIRS and septic shock develop when homeostasis is disrupted and are associated with overproduction of proinflammatory cytokines i. Cytokine cascade: Triggering actions of LPS not fully understood; bacterial products stimulate the production of cytokines by macrophages and monocytes i. Cytokine release begins with the release of tumor necrosis factor-α (TNF-α) followed by the release of IL-1 and IL-6 ii. TNF-α and IL-1 cause a variety of physiologic effects; interferon-γ, released from natural killer cells in response to TNF and bacterial products, amplifies the functions of IL-1 and TNF-α (a) Increased temperature set point, which causes fever or may cause hypothermia (b) Decreased systemic vascular resistance and increased capillary permeability iii. IL-6 is released from T cells and monocytes after tissue injury and may inhibit the release of other cytokines (TNF-α, IL-1); IL-6 stimulates the liver to release chemical mediators known as acute-phase proteins (i.e., C-reactive protein [CRP]) (a) CRP levels increase in tissue-damaging infections (1) CRP binds to the C-polysaccharide cell wall complement found in bacteria and fungi (2) Complement system is activated following the binding of CRP to the cell wall component, which leads to a complement-mediated increase in phagocytosis (b) Increased serum concentrations of CRP are associated with sepsis and death iv. Effects of cytokines are mediated at target tissues by nitric oxide, arachidonic acid metabolites (prostaglandins, eicosanoids, platelet-activating factor), and lipoxygenase derivatives v. TNF-α and IL-1 stimulate the production of other cytokines, which leads to a cascade effect with complex amplification and modulation (upregulation and downregulation) (a) IL-8 induces chemotaxis of activated polymorphonuclear leukocytes and acts as an inflammatory mediator, which leads to tissue damage (b) TNF-α and IL-1 activate the coagulation cascade (1) Bacterial products, TNF-α, and IL-1 induce intravascular coagulation and fibrin deposits (2) Thromboplastin (factor III) and factor VIII activate the extrinsic coagulation pathway (3) Factor XII (Hageman factor) activates the intrinsic coagulation pathway (c) TNF-α and IL-1 activate the complement cascade by factor XII and bacterial products (1) C5a component (one of more than 20 proteins involved in the complement cascade) is a vasoactive anaphylatoxin that binds to macrophages and monocytes (2) Complement stimulates an oxidative burst (3) Complement causes the release of oxygen radicals and proteases that damage cells, particularly type II pneumonocytes in the lungs (4) Complement enhances adherence to the endothelium with degranulation (emptying out granules with digestive substances) and aggregation (clumping), which leads to microvasculature damage (5) C5a binds to mast cells, basophils, and platelets; causes the release of histamine, serotonin, prostaglandins, and leukotrienes; results in vessel dilation, increased blood flow, increased capillary permeability, and increased plasma leakage (d) TNF-α and IL-1 activate the kinin cascade with the production of bradykinin 5. Role of microbial translocation: Controversial theory in humans; describes the passage of microbes such as normal bacterial flora of the gut or of microbial products across an injured intestinal mucosal wall from the gut lumen to the mesenteric lymph nodes, other organs, and blood stream; may be a major contributor to the development of the septic cascade a. Common enteric organisms: Enterococcus, Escherichia coli, Clostridium perfringens, Enterobacter cloacae b. Conditions thought to increase gut permeability and microbial translocation i. Mucosal ischemia and mucosal hypoperfusion caused by shock or mesenteric vasoconstriction from intense sympathetic nervous system (SNS) stimulation ii. Immunoglobulin A deficit associated with total parenteral nutrition, thermal injury, glucocorticoid administration, and endotoxin release iii. Glutamine and fiber deficiencies c. Conditions that contribute to microbial translocation include obstructive jaundice, burns, endotoxemia, hemorrhage, immunosuppression, malnutrition, ischemia, reperfusion injury, total parenteral nutrition, and antibiotic therapy a. Most common sites of origin of bacteremia and sepsis b. Most common organisms in hospitalized patients: Gram-negative aerobes c. Other gram-negative aerobes: Enterobacter and Proteus d. Infections with gram-positive organism are becoming more common because these organisms are associated with use of intravascular catheters and invasive devices. Most common aerobic organisms are the following: f. Predisposing factors for the development of bacteremia or sepsis i. Extremes of age: Elderly and very young iii. Prior antimicrobial therapy iv. Severe burn injury, recent trauma, recent surgical procedures, and invasive procedures vi. Immunosuppression: Infection with human immunodeficiency virus (HIV), chemotherapy, corticosteroids, and bone marrow suppression vii. Malnutrition and total parenteral nutrition viii. Alcohol use and abuse; abuse of other drugs a. Patient health history: Significant past medical and surgical history, with a review of all major systems and the identification of recent invasive procedures and recent travel history i. History of chronic disease (diabetes mellitus; alcoholism; and liver, heart, and renal failure) places the patient at risk ii. Acute illness: Trauma, burns, cholelithiasis, intestinal obstruction, pancreatitis, appendicitis, peritonitis, diverticulitis b. Family health history: Chronic disease or infections c. Social history: Significant others, ability of the patient and significant others to manage stress, financial obligations of the patient and significant others, and parenting responsibilities of the patient d. Medication history, especially medications with immunosuppressive properties (chemotherapeutic drugs, corticosteroids) and antibiotics e. Nutritional history, with a special focus on the causes of primary malnutrition (anorexia nervosa, alcohol abuse) and secondary malnutrition (iatrogenic malnutrition, surgical malnutrition) 2. Nursing examination of patient i. Inspection: Clinical presentation may vary, depending on the patient’s underlying health and organ function (a) Acute distress with anxiety, restlessness, confusion, and disorientation progressing to unresponsiveness (b) Flushed, warm, dry skin or pale, cold, mottled skin (particularly in the elderly), decreased capillary refill; shaking chills and shivering in some patients (d) Decreased urinary output; significant edema or positive fluid balance (a) Tachycardia with rapid, weak, and thready peripheral pulses. Initially, pulses may be bounding and rapid with a hyperdynamic state. (b) Warm skin (elderly may present with cool skin rather than hyperthermia) iii. Percussion: Dullness over areas of consolidation i. Core temperature: Above 100.4° F (38° C) or below 96.8° F (36° C) ii. Heart rate above 90 beats/min iii. Respiratory rate: Higher than 30 breaths/min iv. Blood pressure (BP): Below 90 mm Hg systolic or v. Pain is the fifth vital sign (see Multisystem Trauma) (a) Cardiac index: More than 3.5 L/min/m2; may be low in elderly patients and those with underlying cardiac disorders, or in those in the stagnant phase of septic shock (b) Systemic vascular resistance: Below 900 dynes/sec/cm−5 (c) Pulmonary artery wedge pressure (PAWP): Normal to low; below 6 mm Hg (d) Oxygen delivery and consumption: Variable, but often decreased as septic shock progresses; oxygen extraction below 20% of oxygen delivery (normally 20% to 25%) (1) Experts suggest that the designations of “warm shock” and “cold shock” be abandoned and that the extent of tissue perfusion be used to determine the extent of shock (2) If derived hemodynamic variables (such as oxygen delivery and consumption) are not available, lactate levels or base deficit can be used (see Diagnostic Studies) 3. Appraisal of patient characteristics: A 55-year-old male factory worker is admitted to the hospital with perforated colonic diverticula. He complains of a 2-day history of fever (102° F [38.9° C]), nausea, vomiting, distended abdomen, and abdominal pain, which worsened over the last 6 hours. Patient takes albuterol and metaproterenol for asthma and recently started taking oral steroids for an acute asthma attack. Wife reports that the patient has had a severe cough with production of green sputum and was not taking antibiotics. After diagnosis via computerized tomographic (CT) scan, the patient is taken to the operating room to undergo a Hartmann procedure. Antibiotics, intravenous (IV) fluids, and mechanical ventilation were initiated within 1 hour of admission directly to the ICU. Hemodynamic values are consistent with a hyperdynamic state and WBC count of 10,000/mm3. Family members are at the bedside and provide much support for the patient. They have arranged for assistance with medical technology from a neighbor who works as a critical care registered nurse. a. Resiliency (level 3—moderately resilient): Patient comes to the hospital with SIRS and sepsis; demonstrates strong reserves and the ability to mount an immune response as manifested by fever and hyperdynamic state; is maintaining stable vital signs; the WBC count must be followed to verify his ability to maintain an immune response despite the recent initiation of oral steroid therapy b. Vulnerability (level 1—highly vulnerable): Patient has had definitive resuscitation and treatment for an infectious-inflammatory process, but with the recent history of a productive cough is highly prone to hospital-associated infections c. Stability (level 3—moderately stable): Patient is hemodynamically stable, yet in a hyperdynamic state with a requirement for large volume replacement; stability potential would be limited with the removal of interventions d. Complexity (level 1—highly complex): Based on acute onset, multiple intensive therapies; preexisting symptoms of probable respiratory infection; altered body image due to surgical incision; need for strong family support e. Resource availability (level 5—many resources): Financial resources readily available from stable employment and benefit package; the patient’s knowledge is limited, but he has a neighbor with 20 years’ experience as a critical care nurse and extended family readily available to assist with his care f. Participation in care (level 5—full participation): As previously g. Participation in decision making (level 3—moderate level of participation): Yet to be determined for this patient; the family has the ability to seek assistance from the neighbor h. Predictability (level 3—moderately predictable): Based on symptom onset and presentation for intervention, and age i. Arterial blood gas (ABG) levels (a) Respiratory alkalosis with Paco2 below 32 mm Hg attempting to compensate for metabolic acidosis, with pH below 7.35 and decreased HCO3 (b) Late: Respiratory acidosis with Paco2 above 45 mm Hg (c) Progressive intrapulmonary shunt, with an increasing fraction of inspired oxygen (FIo2) needed to maintain arterial partial pressure of oxygen (Pao2) at 70 mm Hg and oxygen saturation by pulse oximetry above 92% ii. Mixed venous blood gas levels, arterial lactate level, base deficit (a) Increasing hemoglobin saturation of mixed venous blood (S (b) Increasing arterial lactate level (>2 mEq/L) as cells use anaerobic rather than aerobic pathways for metabolism iii. Complete blood count (CBC) and differential: Either increased (>12,000/mm3) or decreased (<4000/mm3) or above 10% immature (band) forms iv. Serum glucose levels: Elevated from the stress response v. Blood cultures and antibiotic sensitivities (a) Identify causative organisms; blood culture results are positive in only 50% of septic patients for uncertain reasons (bacteremia may be intermittent) (b) Urine, sputum, and wound cultures to correlate with blood cultures vi. Elevated blood urea nitrogen (BUN) and creatinine levels vii. Coagulation studies: May show elevations in prothrombin time (PT) and partial thromboplastin time (PTT); decreased fibrinogen level and increased level of fibrin split products viii. Decreased platelet levels ix. Decreased CRP and elevated procalcitonin levels x. Elevated serum enzyme levels, indicating liver or cardiac impairment 1. Infection and exaggerated inflammatory process i. Exaggerated or “malignant” inflammation ii. Inadequate primary defenses (broken skin, traumatized tissues) and secondary defenses (immunosuppression), invasive procedures, and/or malnutrition i. WBC count is 4000 to 12,000/mm3 ii. Temperature is 96.8° to 100.4° F (36° to 38° C) iii. Heart rate is 60 to 100 beats/min c. Collaborating professionals on health care team: Registered nurse, medical/surgical attending physician, respiratory therapist, pharmacist, dietitian, infectious disease specialist, pastoral counselor i. Administer antimicrobial agents on time ii. Monitor antibiotic levels, particularly aminoglycoside levels, for renal and ototoxic effects iii. Monitor for reaction to antibiotics (a) Superinfection: Infection with organisms such as C. albicans is usually controlled by normal body flora (b) Allergy: Rash and anaphylactic shock (c) Resistance: Reemergence of symptoms of fever, purulence, and increased WBC count iv. Monitor compliance with unit infection control protocols, as recommended by the Centers for Disease Control and Prevention v. Drotrecogin alfa (activated) is recombinant human activated protein C; indicated for the treatment of severe sepsis in adult patients with a high risk of mortality (a) Evaluate all patients for possible drotrecogin alfa (activated) therapy if receiving an antibiotic and vasopressor (b) Drug properties: Antithrombotic, antiinflammatory, and profibrinolytic (c) Continuous intravenous infusion at a dose of 24 mcg/kg/hr for a total of 96 hours (d) Side effect: Bleeding; if it occurs, the physician should be notified immediately (e) Discontinue administration of the drug 2 hours before a surgical procedure and restart once adequate hemostasis is achieved (usually within 12 hours after the procedure) vi. Corticosteroids may be administered to decrease inflammation, improve vessel reactivity to vasopressor agents, decrease the time the patient requires vasopressors, and improve patient outcome (a) An adrenocorticotropic hormone (ACTH) test should precede therapy. Treatment should be continued only in patients with corticosteroid insufficiency (randomly measured cortisol level of 15 mcg/dl or less, peak cortisol level of 20 mcg/dl or less, or a cortisol increment of 9 mcg/dl or less). (b) Dosage: Hydrocortisone 200 to 300 mg IV and fludrocortisone 50 mcg orally daily may be given for 1 week from the onset of shock (defined by vasopressor requirement) vii. Provide twice-a-day brushing of the teeth, oral cleansing every 2 hours, and suctioning above the endotracheal tube viii. Assist with treatments to limit the nidus of infection ix. Stabilize fractures promptly to limit tissue damage and inflammation x. Maintain strong rapport with the family and provide frequent updates and education, because the course of this disease is often unpredictable 2. Maldistribution of blood flow (renal, cerebral, cardiopulmonary, gastrointestinal, peripheral) i. Hyperdynamic state with increased cardiac output and index is the usual presentation ii. Subsequently, hypovolemia develops iii. Arterial lactate levels begin to increase iv. Inability of the tissues to use oxygen; S vi. Decreased gastric mucosal pH showing cellular acidosis vii. Diminished bowel sounds and/or paralytic ileus viii. Excessive microvascular coagulation and impaired fibrinolysis ix. Decreased systemic vascular resistance and hypotension (systolic BP below 90 mm Hg) x. Changes in the sensorium (restlessness, anxiety, and disorientation progressing to unresponsiveness) i. Oxygen delivery and consumption are normal or supranormal ii. S iii. Arterial lactate levels are normal iv. Urine output is at least 1 ml/kg/hr v. Systolic BP is above 90 mm Hg, and systemic vascular resistance is normal vi. Bowel sounds are present and there is no abdominal distention vii. Sensorium is clear; the patient is oriented to time, place, and person c. Collaborating professionals on health care team: See Infection and Exaggerated Inflammatory Process i. Monitor hemodynamic parameters and S ii. Be prepared to administer fluid resuscitation (a) Suggested possible end point is a PAWP of 12 mm Hg but it varies, depending on the patient’s underlying condition (b) Type of fluid to be used (colloid versus crystalloid) is controversial; blood transfusion may be needed if the hemoglobin level is less than 10 mg/dl iii. See interventions for hypovolemic shock iv. Be prepared to administer vasoactive medications as needed if fluid resuscitation fails to maintain BP and organ perfusion (a) Combined inotropic agent and vasopressor may be used (1) Norepinephrine, 1 to 30 mcg/min (2) Phenylephrine, 40 to 180 mcg/min (3) Vasopressin, 0.01 to 0.04 units/min; appropriate in patients requiring high-dose vasopressors (4) Dopamine (6 to 25 mcg/kg/min) and epinephrine (1 to 10 mcg/min) may also be used; may induce or exacerbate tachycardia (b) Early goal-directed therapy (EGDT); involves maximizing cardiac preload, afterload, and contractility to balance oxygen delivery with demand (Rivers, Nguyen, Havstad, et al, 2001) (1) Patient monitoring is necessary with arterial line and central venous oxygen saturation (ScVo2) (2) Goal of patient therapy: Central venous pressure (CVP) of 8 to 12 mm Hg, maintained with 500-ml crystalloid bolus every 30 minutes; mean arterial pressure between 65 and 90 mm Hg, maintained with vasoactive agents; ScVo2 of 70% or higher, maintained with transfusion of red cells to sustain a hematocrit of 30% or higher and inotropic agents. Goal is to complete resuscitation within 6 hours of diagnosing sepsis. (3) Among septic patients receiving EGDT, in-hospital mortality was reduced by 34% and hospital length of stay declined by 3.8 days v. Monitor for symptoms of diminished visceral perfusion vi. Avoid Trendelenburg’s position, which may impair gas exchange and decrease cerebral perfusion vii. Maximize oxygen delivery and utilization; minimize oxygen demand (1) Use tepid baths or a cooling blanket (2) Prevent chills and shivering (4) Use antipyretic agents other than aspirin to reduce fever (b) Reduce the work of breathing with mechanical ventilation, as appropriate; work with physicians to sedate and paralyze the patient, as needed, to maintain adequate ventilation and gas exchange viii. Limit patient activity; maintain a restful environment; provide uninterrupted rest; maintain family visitations as appropriate ix. Manage pain, anxiety, and restlessness with medications and nursing interventions x. Administer medications if appropriate to modify mediators (Table 9-2) 3. Impaired oxygenation and ventilation: See Chapter 2 i. Related to the body’s response to infection and the inflammatory process ii. Core temperature below 96.8° F (36° C) or above 100.4° F (38° C) iii. Flushed, warm skin or pale, cool skin c. Collaborating professionals on health care team: See Infection and Exaggerated Inflammatory Process i. Monitor core temperature hourly (a) Pulmonary artery (PA) thermistor is the instrument of choice (b) If no PA catheter is in place, use the rectal, urinary bladder, esophageal, or tympanic route ii. After the source of increased or decreased temperature is identified, maintain normothermia by the use of antipyretic medication as prescribed; avoid aspirin products iii. Use tepid baths or a cooling blanket to reduce hyperthermia (a) Monitor core temperature at all times to reduce the risk of hypothermia (b) Do not decrease temperature too rapidly, because this may lead to shaking chills (c) Reposition frequently, and check for tissue breakdown if a cooling blanket is used iv. Use warming blankets and a warmed ambient temperature to manage hypothermia 5. Catabolic state resulting in malnutrition c. Collaborating professionals on health care team: See Infection and Exaggerated Inflammatory Process d. Interventions: See Chapter 8 i. Initiate enteral feedings within 48 hours to limit gastrointestinal microbial translocation ii. Establish caloric requirements based on body size and degree of hypermetabolism; 20 to 25 kcal/kg/day is average iii. Maintain glucose level at 80 to 110 mg/dl because hyperglycemia is associated with a poor prognosis iv. Provide family/significant others with distinct goals for nutritional support 1. Definition developed by a consensus conference and reported in Bone, Balk, and Cerra, et al (1992): Multiple organ dysfunction syndrome (MODS) is the presence of altered organ function in an acutely ill patient such that homeostasis cannot be maintained without intervention. Other terms used are the following:
Multisystem
SYSTEMWIDE ELEMENTS
Patient Assessment
 o2) above 80% as tissues are unable to extract delivered oxygen
o2) above 80% as tissues are unable to extract delivered oxygen
Patient Care
 o2 above 80%; oxygen extraction ratio below 20%
o2 above 80%; oxygen extraction ratio below 20%
 o2 is 65% to 75%, and oxygen extraction ratio is improved (>20%)
o2 is 65% to 75%, and oxygen extraction ratio is improved (>20%)
 o2 along with derived parameters, such as systemic vascular resistance, oxygen delivery, and oxygen consumption
o2 along with derived parameters, such as systemic vascular resistance, oxygen delivery, and oxygen consumption
SYSTEMWIDE ELEMENTS
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Multisystem
Get Clinical Tree app for offline access