Multiple Gestation
Nancy A. Bowers
During the past 30 years, multiple birth rates have increased dramatically, with the recent numbers of twins, triplets, and other higher order multiples (HOMs) at the highest levels in recorded history. Trends in delayed childbearing and increased use of infertility therapies and assisted reproductive technologies (ARTs) have contributed greatly to these increased rates (Wright, Schieve, Reynolds, & Jeng, 2005). Although multiple births represent a little more than 3% of the total live births in the United States, these births contribute disproportionately to the rates of maternal, fetal, and neonatal morbidity and mortality. Multiple birth infants are at high risk of being born too early and too small and have an impact on national rates of preterm birth (<37 weeks’ gestation) and low birth weight (LBW; less than 2,500 g). A key factor is a clear dose-response relationship: Increasing plurality corresponds with higher morbidity and mortality for mothers and their infants. With these high risks, the perinatal team must be alert for potential complications during pregnancy, labor, birth, and the postpartum period.
EPIDEMIOLOGY
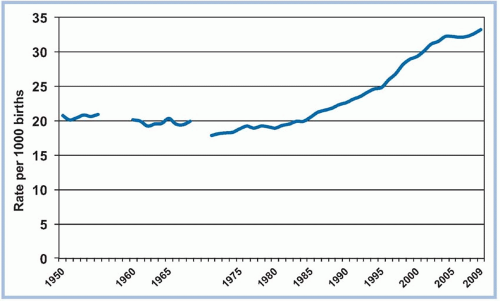
Of the more than four million United States live births in 2009, multiple births accounted for 3.32%, with 137,217 infants born as twins, 5,905 as triplets, 355 as quadruplets, and 80 as quintuplets and higher (Martin et al., 2011). Data from the National Center for Health Statistics (Martin et al., 2011) reveal that the multiple birth rate (live births of twins, triplets, and higher/1,000) remains at record levels (see Figures 11-1 and 11-2). The twin birth rate increased by 76% since 1980 and by 47% since 1990 but has now stabilized, rising about 1% annually (Martin et al., 2011). During that same time, the rate of HOMs rose more than 500% but has declined since 2004 (Martin et al., 2011).
A population-based study of births from 1995 to 2000 found mothers of multiples were more likely to be older, white, of lower parity, nonsmokers, married, and to have higher education than mothers of singletons (Luke & Brown, 2007). Shifts in traditional maternal age patterns have occurred along with the rise in multiple births, mirroring the trend of delayed childbearing. Historically, twin birth rates were low for young women, rose through the age group 35 to 39 years, then declined for women in their 40s (Martin & Park, 1999). However, beginning in 1992, multiple birth rates rose steadily for women over age 30, with the highest overall multiple birth rates in 2009 for women in the 45 to 54 age group (237.3/1,000; Martin et al., 2011). Surprisingly, advanced maternal age appears to be associated with better perinatal outcomes for multiple gestations compared to singletons (Delbaere et al., 2008; Kathiresan et al., 2011). This is particularly true for nulliparous women. A variety of explanations have been proposed, including greater use of donor eggs and better socioeconomic status, education, and prenatal care (Zhang, Meikle, Grainger, & Trumble, 2002). Other explanations include older women’s healthier lifestyles and higher body mass index (BMI), as well as possible uterine cell proliferation or “remodeling” from prior pregnancies that may improve uterine expansion limits and fetal nourishment (Oleszczuk, Keith, & Oleszczuk, 2005).
There have also been shifts in maternal race patterns for multiple births. Historically, multiple births occurred more often in black women than in white women, but this gap has been overtaken. In 2009, twin birth rates for non-Hispanic white women and
non-Hispanic black women were similar (Martin et al., 2011). However, disparities were seen for HOMs. In 2009, the HOM birth rate for non-Hispanic whites (201.4/100,000) was nearly double the rate of non-Hispanic blacks (105.6/100,000) and was even higher than that of Hispanic mothers (83.5/100,000; Martin et al., 2011). Regardless of maternal age, a prior multiple pregnancy and higher parity increase the likelihood for spontaneous dizygotic (DZ) twin conception. Twinning is about four times more likely in the fourth or fifth birth than in the first birth (Taffel, 1995).
non-Hispanic black women were similar (Martin et al., 2011). However, disparities were seen for HOMs. In 2009, the HOM birth rate for non-Hispanic whites (201.4/100,000) was nearly double the rate of non-Hispanic blacks (105.6/100,000) and was even higher than that of Hispanic mothers (83.5/100,000; Martin et al., 2011). Regardless of maternal age, a prior multiple pregnancy and higher parity increase the likelihood for spontaneous dizygotic (DZ) twin conception. Twinning is about four times more likely in the fourth or fifth birth than in the first birth (Taffel, 1995).
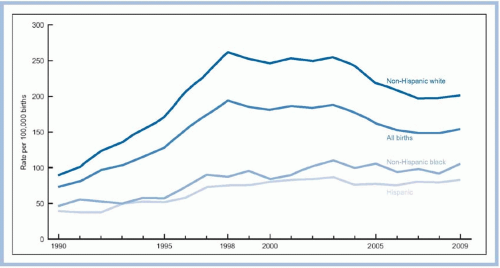 FIGURE 11-2. Higher order multiples birth rate: United States 1990-2009. (From Martin, J. A., Hamilton, B. E., Ventura, S. J., Osterman, M. J. K., Kirmeyer, S., Mathews, T. J., & Wilson, E. C. [2011]. Births: Final data for 2009. National Vital Statistics Reports, 60[1], 1-72. Retrieved from http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_01.pdf. Reprinted with permission.) |
PHYSIOLOGY OF TWINNING
Multiple gestations result from either the fertilization of a single ovum that subsequently divides into two or more zygotes (monozygotic [MZ]) or the fertilization of multiple ova (DZ, trizygotic [TZ], etc.). The spontaneous MZ twinning rate is 0.4%, but the rate with ART procedures is 2 to 12 times higher (Aston, Peterson, & Carrell, 2008). Mechanisms that may contribute to this increase include maternal age, ovarian stimulation, zona manipulation, temperature, and in vitro embryo culture (Aston et al., 2008).
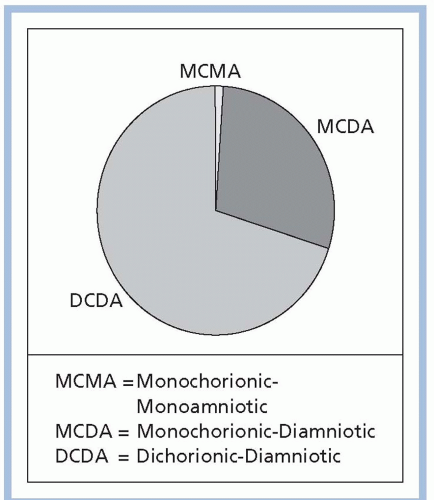
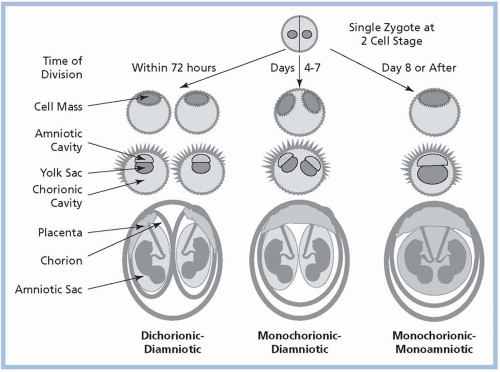
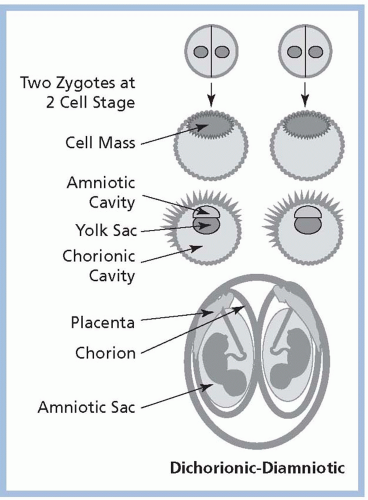
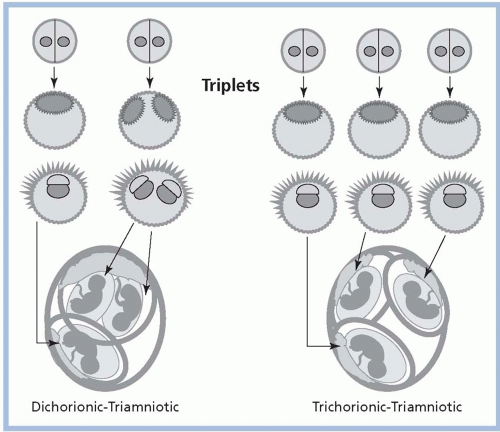
Among MZ twin pregnancies, about 30% are dichorionic/diamniotic (DC/DA; separate placentas and amniotic sacs); about 70% are monochorionic/diamniotic (MC/DA; a single placenta with two amniotic sacs); and about 1% are monochorionic/monoamniotic (MC/MA), in which multiple fetuses share a single placenta and one amniotic sac (Smith-Levitin, Skupski, & Chervenak, 1999; see Fig. 11-3).The degree to which structures are shared is related to the time of zygotic division after conception; early division (within 72 hours) results in DC/DA, division between days 4 through 7 results in MC/DA, and later division results in MC/MA. Conjoined twins occur following very late and incomplete zygotic splitting, usually after day 13. Because they develop from a single fertilized ovum, MZ twins are always of the same gender. DZ twins are always DC/DA and may be the same or different gender. Triplets and other HOMs may have any combination of chorionicity/amnionicity, such as trichorionic/triamniotic (TC/TA), dichorionic/triamniotic (DC/TA), or monochorionic/triamniotic (MC/TA; see Figs. 11-4,11-5 and 11-6).
ROLE OF INFERTILITY AND ASSISTED REPRODUCTIVE TECHNOLOGY
A significant percentage of multiple births in the United States are conceived with some type of infertility therapy,
including ovulation induction and ART. Although data from the National Summary and Fertility Clinic Report from the Centers for Disease Control and Prevention (CDC), the American Society for Reproductive Medicine (ASRM), and the Society for Assisted Reproductive Technology (SART) provide a fairly accurate estimate of in vitro fertilization (IVF) births, exact numbers of natural conceptions are unknown. Of live births from pregnancies conceived in ART cycles using fresh nondonor eggs or embryos in 2009, 28.9% were twins and 1.6% were HOMs (CDC, ASRM, & SART, 2011; see Fig. 11-7). Among ART births in 2009, 60% of twins and 97.5% of HOMs were born preterm, and 56.1% of twins and 92.1% of HOMs were LBW (CDC et al., 2011).
including ovulation induction and ART. Although data from the National Summary and Fertility Clinic Report from the Centers for Disease Control and Prevention (CDC), the American Society for Reproductive Medicine (ASRM), and the Society for Assisted Reproductive Technology (SART) provide a fairly accurate estimate of in vitro fertilization (IVF) births, exact numbers of natural conceptions are unknown. Of live births from pregnancies conceived in ART cycles using fresh nondonor eggs or embryos in 2009, 28.9% were twins and 1.6% were HOMs (CDC, ASRM, & SART, 2011; see Fig. 11-7). Among ART births in 2009, 60% of twins and 97.5% of HOMs were born preterm, and 56.1% of twins and 92.1% of HOMs were LBW (CDC et al., 2011).
Fertility-assisted pregnancies, including singletons, appear to have poorer perinatal outcomes than naturally conceived pregnancies. However, the Report cautions against comparing preterm and LBW births between ART’s multiple-fetus pregnancies with those of the general population since a substantial proportion of twin births or HOM births are due to infertility treatments (ART and non-ART) (CDC et al., 2011).
The high risk of multiple births with ART and other infertility therapies has generated the creation of policy recommendations and clinical guidelines. In 1998, 2004, 2006, 2009, and again in 2013, SART and the ASRM declared that high-order multiple pregnancy (three or more implanted embryos) is an undesirable consequence of ART and issued guidelines to assist ART programs and patients in determining the appropriate number of embryos to transfer (Practice Committee of ASRM & Practice Committee of SART, 2013). The 2013 guidelines include the following maternal age-based embryo transfer limits for women with a favorable prognosis: transfer of one to two cleavage-stage embryos for women under age 35, two cleavage-stage embryos for women between 35 and 37 years of age, three cleavage-stage embryos for women between ages 38 and 40, and no more than five embryos for women ages 41 to 42. Data are insufficient to recommend a limit for women ≥43 years of age. The guidelines may
be individualized based on each woman’s clinical condition, prognosis, and circumstances.
be individualized based on each woman’s clinical condition, prognosis, and circumstances.
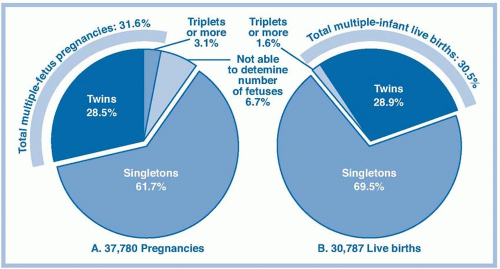 FIGURE 11-7. Risks of having multiple-fetus pregnancies and multiple-infant live births from assisted reproductive technology (ART) cycles using fresh nondonor eggs or embryos, 2009. (From Centers for Disease Control and Prevention, American Society for Reproductive Medicine, & Society for Assisted Reproductive Technology [2011]. 2009 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Atlanta, GA: U.S. Department of Health and Human Services. Retrieved from http://www.cdc.gov/art/ART2009/index.htm. Reprinted with permission.) |
In the early 1990s, other countries instituted governmental mandates for IVF clinics to decrease the number of embryos transferred from three to two (Jain, Missmer, & Hornstein, 2004). By 2001, marked declines were seen in the rate of multiple births after IVF, especially in HOMs. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) reported an overall twin rate of 25.7% per delivery and a triplet rate of 2.5% in 2002 for 53 countries (ICMART et al., 2009). The group reported the following: the percentage of cycles with ≥4 embryos transferred had decreased from the prior report, rates remained quite high in some countries (South Korea [53.7%], Latin America [37.9%], India [37.2%], and the United Arab Emirates [37.2%]), and the lowest rates (0 to 0.5%) were in 11 countries in Europe and Australia. Countries with the greatest proportion of single embryo transfers were Finland (38.5%), Sweden (30.5%), and Australia (25.0%). Importantly, the decline in multiple births appears to have a significant impact on preterm births. Sweden had a 72% decrease in adjusted odds ratio (from 4.63 to 1.33) for preterm births (Källen, Finnstrom, Nygren, & Olausson, 2005).
It appears that the voluntary implementation of embryo transfer limits in U.S. clinics has also reduced the occurrence of multiple births resulting from ART. The percentage of live-born multiple-infant births dropped from 42% in 1998 to 35% in 2009 for women <35 years old. For women aged 41 to 42, the percentage of multiple-infant live births decreased from 20% to 16% in those same years (CDC et al., 2011).
A continued decrease in the number of iatrogenic multiple births is challenging. Infertility practices are often motivated by competition for patients, the desire for fertility success, and the need for rapid results (D’Alton, 2004). Infertility patients themselves often desire a multiple pregnancy. A review of the recent literature found that while the majority of IVF patients and their partners showed a desire for twins rather than singletons, elective single embryo transfer could become increasingly acceptable if success rates approached those of double embryo transfers (Leese & Denton, 2010). A Cochrane review (Pandian, Bhattacharya, Ozturk, Serour, & Templeton, 2009) concluded that elective single embryo transfer does result in fewer multiple pregnancies than double embryo transfer, but the pregnancy and live birth rate per fresh IVF cycle was lower. The Review did show that the cumulative live birth rate associated with single embryo transfer when followed by a single frozen and thawed embryo transfer was comparable with that after one cycle of fresh, double embryo transfer yet maintained a lower rate of multiple births. A randomized controlled trial that compared elective single embryo transfer to double embryo transfer of blastocyst stage embryos found no statistical difference in pregnancy rate and a reduction in multiple gestation rate from 47% to 0% (Gardner et al., 2004).
MULTIFETAL PREGNANCY REDUCTION
Multifetal pregnancy reduction (MFPR) is a pregnancy termination procedure that reduces the number of fetuses in a HOM pregnancy to a lower and potentially
more viable number, thus increasing the chances for a positive pregnancy outcome. The rise in use of MFPR has paralleled the rising number of HOM pregnancies, particularly in older women using donor eggs (Evans & Britt, 2010). MFPR is typically performed in the late first trimester to terminate one or more fetuses. Nearly all procedures performed today use transabdominal needle insertion under ultrasound guidance. The patient is placed in the lithotomy position, and a 22-gauge needle is inserted transabdominally into the fetal heart. Asystole is achieved following injection of 2 to 3 mL of potassium chloride and is confirmed by ultrasound. Antibiotics are given, and the woman may have abdominal cramping, vaginal spotting, and leaking of amniotic fluid (Little, 2010). Although MFPR is sometimes called selective reduction, the terms are not synonymous. Selective reduction applies to situations in which a specific fetus is targeted for reduction because of a known anomaly, aneuploidy, or a risk identified with nuchal translucency (NT) screening. Reduction of a single fetus in an MC pair requires occlusion of umbilical cord blood flow to prevent interfetal transfusion. Recently, radio frequency ablation has been used to destroy cord tissue for selective reduction of an MC twin (Paramasivam et al., 2010).
more viable number, thus increasing the chances for a positive pregnancy outcome. The rise in use of MFPR has paralleled the rising number of HOM pregnancies, particularly in older women using donor eggs (Evans & Britt, 2010). MFPR is typically performed in the late first trimester to terminate one or more fetuses. Nearly all procedures performed today use transabdominal needle insertion under ultrasound guidance. The patient is placed in the lithotomy position, and a 22-gauge needle is inserted transabdominally into the fetal heart. Asystole is achieved following injection of 2 to 3 mL of potassium chloride and is confirmed by ultrasound. Antibiotics are given, and the woman may have abdominal cramping, vaginal spotting, and leaking of amniotic fluid (Little, 2010). Although MFPR is sometimes called selective reduction, the terms are not synonymous. Selective reduction applies to situations in which a specific fetus is targeted for reduction because of a known anomaly, aneuploidy, or a risk identified with nuchal translucency (NT) screening. Reduction of a single fetus in an MC pair requires occlusion of umbilical cord blood flow to prevent interfetal transfusion. Recently, radio frequency ablation has been used to destroy cord tissue for selective reduction of an MC twin (Paramasivam et al., 2010).
There have been many studies comparing pregnancy outcomes following MFPR. The literature is conflicting regarding the outcomes of reduced and unreduced triplets (Wimalasundera, 2010), and a 2009 Cochrane review reconfirmed that there are “insufficient data available to support a policy of pregnancy reduction procedures for women with a triplet or higher order multiple pregnancy” (Dodd & Crowther, 2010). Generally, outcomes following reduction to twins appear comparable to outcomes for twins conceived spontaneously or by ART, but study findings about the risks and benefits associated with reduction procedures are not as clear (Dodd & Crowther, 2005). Complications include procedural complications (e.g., infection or incomplete procedure), total pregnancy loss, and a continued risk for preterm birth. Collaborative data from several centers have shown that higher starting numbers of fetuses (≥6) correspond with poorer outcomes after reduction; total pregnancy loss rates prior to 24 weeks’ gestation are 4.5% for triplets, 8% for quadruplets, 11% for quintuplets, and 15% for sextuplets or more (Evans, Ciorica, Britt, & Fletcher, 2005). All reduced pregnancies have been shown to have an ongoing increased risk for preterm birth, except for pregnancies reduced to singletons (Evans et al., 2005). Generally, there is an increased risk for lower birth weights in the remaining fetuses post-MFPR, with an inverse correlation to starting numbers (American College of Obstetricians and Gynecologists [ACOG], 2007; Stone et al., 2008). Without prenatal diagnostic testing prior to MFPR, there is the possibility of terminating a healthy fetus while leaving an abnormal one. Some centers are combining the use of rapid chromosome analysis following chorionic villus sampling (CVS) with MFPR (Evans & Britt, 2010). The ASRM recommends that MFPR should be performed only in specialized centers with fetal medicine practitioners experienced in the procedure (ASRM, 2012).
MFPR presents a difficult dilemma for most couples. They often face conflicting values as they consider reduction after years of desiring fertility and must weigh the medical/obstetric/neonatal risks and the psychosocial/moral/economic impact on their family (Elliott, 2005a). The seemingly (and often actually) arbitrary choice as to which fetus should live and which should die is distressing (Bryan, 2005). Further, the decision for reduction must be made in a short time between diagnosis and the optimal timing for the procedure. The decision has been described as highly stressful, psychologically traumatic, frightening, painful, overwhelming, confusing, and a surreal experience (Bergh, Möller, Nilsson, & Wikland, 1999; Bryan, 2005; Collopy, 2004). The following responses from women indicate their inner conflicts about the decision: “I believe reduction saved one of my children. It’s the not knowing that kills me.” “If I tried to carry all of the babies, I would most definitely lose them all. I also look at my survivor knowing she probably would have been chosen for reduction. This also wracks me with guilt” (from a mother who spontaneously lost some of her fetuses and did not have to reduce) (Pector, 2004, p. 716). There is also evidence from interviews with patients that viewing fetuses on ultrasound just prior to or during reduction made the reduction more difficult (Maifeld, Hahn, Titler, & Mullen, 2003; Schreiner-Engel, Walther, Mindes, Lynch, & Berkowitz, 1995). Studies have shown that persistent feelings of sadness and guilt may continue after the MFPR procedure; however, most women believe their choice was correct and that reduction was necessary for them to achieve their goal of motherhood (Collopy, 2004). The grief response with MFPR may not fit the classic process and may be more complicated or delayed. Wang and Yu Chao (2006) interviewed six Taiwanese women undergoing MFPR over 8 to 10 weeks. Although the women were able to move past the reduction and adjust to a normal pregnancy experience, the researchers found that MFPR created psychological distress through exposure to moral and ethical dilemmas. More research is needed to determine long-term psychological outcomes, cultural responses, and effects on parent-child bonding and responses of child survivors.
The reduction debate also can be problematic for clinicians. With the improvements in neonatal care and long-term outcomes for preterm infants, some clinicians may be unwilling to accept that medical risks
are sufficient grounds for reducing a triplet pregnancy (Bryan, 2005). Another consideration is the first trimester spontaneous loss rate for multiple gestations, which might make MFPR unnecessary. One study found that spontaneous loss of one or more gestational sacs or embryos occurred before the 12th week of gestation in 65% of quadruplet pregnancies, 53% of triplets, and 36% of twins (Dickey et al., 2002). The controversy is likely to continue. Some have proposed that future debates will no longer be over reduction of HOMs and triplets but over a routine offer of reduction of all twins (Evans & Britt, 2010). ACOG concludes that patients should not be given the impression that multifetal pregnancies are without problems because fetal reduction is available (ACOG, 2007).
are sufficient grounds for reducing a triplet pregnancy (Bryan, 2005). Another consideration is the first trimester spontaneous loss rate for multiple gestations, which might make MFPR unnecessary. One study found that spontaneous loss of one or more gestational sacs or embryos occurred before the 12th week of gestation in 65% of quadruplet pregnancies, 53% of triplets, and 36% of twins (Dickey et al., 2002). The controversy is likely to continue. Some have proposed that future debates will no longer be over reduction of HOMs and triplets but over a routine offer of reduction of all twins (Evans & Britt, 2010). ACOG concludes that patients should not be given the impression that multifetal pregnancies are without problems because fetal reduction is available (ACOG, 2007).
Nurses have much to contribute in the care of women considering and undergoing MFPR. Establishing a rapport, exploring treatment options, and assisting with decision making with a consistently available primary nurse has been recommended (Collopy, 2004). Patient education includes written instructions, a list of symptoms that would indicate a need for medical care, postprocedure self-care, and grief counseling (Little, 2010).
DIAGNOSIS OF MULTIPLE GESTATIONS
First trimester ultrasound is highly accurate in diagnosing multiple gestations; the earliest gestation for determining chorionicity is 5 gestational weeks; for fetal number, 6 weeks; and for amnionicity, 8 weeks (Shetty & Smith, 2005). Diagnosis is confirmed when multiple embryos or embryonic parts are seen in the gestational sac(s). Women at high risk of conceiving multiples, such as those using infertility therapies, should have an ultrasound early in the first trimester. Thickness and numbers of membrane layers can be counted, and ultrasound markers can be visualized between 6 and 10 gestational weeks (Cleary-Goldman, Chitkara, & Berkowitz, 2007). Membrane thickness of ≤2 mm identifies monochorionicity with a positive predictive value of 90%, and >2 mm identifies dichorionicity with a positive predictive value of 95% (Morin & Lim, 2011). The triangular lambda sign predicts DZ chorionicity, a T-shaped junction is present in MC placentas, and a Y-shaped ipsilon zone is characteristic of the three interfetal membranes of TZ triplet gestations (Shetty & Smith, 2005; Smith-Levitin et al., 1999). First trimester ultrasonography is highly accurate in determining chorionicity, but accuracy decreases as the pregnancy progresses, especially after 14 weeks (Morin & Lim, 2011), and varies with sonographer skill and experience (Menon, 2005). Early identification of MC and MA pregnancies is important in planning appropriate management of these high-risk pregnancies.
Clinical examination may also assist in diagnosis. With an accurate menstrual history, a fundal height 2 cm to 4 cm larger than the expected gestational age suggests multiple gestation. Other signs include palpation during the Leopold maneuver that reveals multiple fetal parts or fetal poles, or when there is more than one fetal heart sound, particularly with a difference of 10 beats per minute (Bowers & Gromada, 2006).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access