SIGNIFICANCE AND INCIDENCE
Preterm birth (PTB) is birth occurring before 37 completed weeks of gestation. Based on the most recent data available, in 2010, the PTB rate in the United States was approximately 12% (
Hamilton, Martin, & Ventura, 2011). This rate reflects an increase of 30% between 1981 and 2006. Although there was a slight decline in the PTB rate between 2007 and 2010, it remains higher than any year in the period between 1981 and 2006 (
Martin, 2011;
Martin, Osterman, & Sutton, 2010). For the last two decades, the largest contribution to the increase in PTBs was late PTBs between 34 and 36 completed weeks of gestation (
Raju, Higgins, Stark, & Leveno, 2006). From 1992 to 2002, two thirds of the increase in the rate of PTBs in the United States was in this subset of preterm babies (
Davidoff et al., 2006). The small drop in the PTB rate for 2010 was primarily among babies born late preterm, which decreased 2% from 8.66% in 2009 to 8.49%. The 2010 late preterm rate was 7%, lower than the 2006 high (9.14%). The 2010 rate for early preterm births (less than 34 weeks of gestation) was essentially stable at 3.50%, with 1.53% between 32 and 33 weeks and 1.97% less than 32 weeks (
Hamilton et al., 2011). In 2010, late PTBs continued to comprise two thirds of the total PTBs, with 8.5% late preterm and 12% total preterm (
Hamilton et al., 2011).
The
Institute of Medicine (IOM; 2007) report on PTB cited “troubling and persistent disparities in PTB rates among different ethnic and racial groups” (
p. 1). The highest rates of PTB are among non-Hispanic blacks (17.15% in 2010). Although this is the lowest PTB rate for non-Hispanic blacks in over three decades, it is still 46% higher than Hispanic infants and 59% higher than non-Hispanic white infants (
Hamilton et al., 2011;
Martin et al., 2011). Other racial and ethnic groups have lower rates, but all are still unacceptably high. The rate of PTB for American Indians is 13.9%; for Hispanics, 11.97%; for non-Hispanic whites, 10.9%; and for Asians, 10.9%. PTB is the leading source of neonatal mortality and morbidity in the United States (
IOM, 2007); thus, PTB is a problem that has attracted decades of research in an attempt to discover possible causes and cures.
The rising rates of PTB in the United States contrast with the drop in rates of infant mortality since the 1950s. In 2007, the rate of infant mortality for the United States was 6.75%; in 1950, by comparison, it was 29.2% (
MacDorman & Mathews, 2011;
March of Dimes, 2012a). This dramatic change occurred because of the emergence of the science of neonatal care, with more babies now living past their first birthday despite being born at earlier gestational ages. Sophisticated neonatal care has also allowed more preterm babies to survive, although for many of the smallest preterm babies, that survival is liable to come with significant morbidity, which could last throughout their lives.
It is important when discussing PTB that everyone uses the same definitions. Although they are different entities, often with separate etiologies, the terms
preterm birth and
low birth weight (LBW) are commonly used interchangeably. In fact, most of the long-term follow-up studies of children and adults quoted in the literature are of very-low-birth weight (VLBW) or extremely-low-birth weight (ELBW) children because assessment of gestational age was not common in perinatal care until recent decades. Birth weight has been, and continues to be, a simple and definitive assessment,
thus easier to access. PTB refers only to gestational age at birth, no matter the birth weight. Although the term
preterm birth (< 37 weeks) is commonly used, other classifications in the literature of babies born preterm include moderately preterm (32 to 34 weeks), late preterm (34 to 36 weeks), and very PTB (< 32 weeks). Preterm babies may be, but do not have to be, low birth weight. A preterm baby born to a mother with gestational diabetes, for instance, might be of normal birth weight and yet be born preterm. The prematurity would then dictate the health problems of the baby, as lung, central nervous system, or gastrointestinal immaturity would pose health risks.
LBW refers only to weight at birth, no matter the gestational age. A baby is considered LBW if it is born <2,500 g (5.5 lb). VLBW is defined as birth <1,500 g (3.5 lb), and ELBW is <1,000 g (<2.2 lb). LBW babies may be born before 37 weeks but can also be born at term (e.g., a baby at 41 weeks gestational age could weigh 1,800 g because of intrauterine growth restriction [IUGR]). Risk factors, causes, and outcomes for LBW, growth restriction, and PTB are interrelated but can cause some confusion when reviewing the literature.
The rates of LBW and PTB in the United States are different. The LBW rate increased more than 20% from the mid-1980s through 2006 but has trended slightly downward since. In 2010, the LBW rate was 8.15%. The rate of VLBW was 1.45% in 2010, unchanged from 2009. The VLBW rate increased during the 1980s and 1990s, peaking at 1.49% in 2007, but declined to 1.45% to 1.46% for 2008 to 2010 (
Hamilton et al., 2011).
Much research has been conducted on the sequelae of LBW, VLBW, and ELBW. Babies with the most mortality and morbidity are the VLBW and ELBW babies. A baby born at VLBW (<1,500 g) is at high risk for neonatal mortality (
De Jesus et al., 2012;
Morales et al., 2005) and, when compared with normal birth weight children, is at higher risk for learning disabilities and cognitive deficiencies during childhood (
Litt, Taylor, Klein, & Hack, 2005;
Orchinik et al., 2011). Low gestational age is a corresponding risk factor for neonatal morbidity. Although the majority of babies with gestational ages of greater than or equal to 24 weeks survive, there are high rates of morbidity among survivors. In a study of 9,575 babies of extremely low gestational age (22 to 28 weeks) and VLBW or ELBW (401 to 1,500 g), babies at the lowest gestational ages were at greatest risk for morbidities (
Stoll et al., 2010). Overall, 93% had respiratory distress syndrome, 46% had patent ductus arteriosus, 16% had severe intraventricular hemorrhage, 11% had necrotizing enterocolitis, 36% had late-onset sepsis, and 68% had bronchopulmonary dysplasia with greater than 50% undetermined retinopathy status at the time of discharge from the neonatal intensive care unit (NICU).
VLBW children followed through adulthood have been found to have poorer educational achievement, higher blood pressures, poorer respiratory function, and generally poorer physical abilities than their peers who were born at normal birth weight (
Hack, 2006). ELBW babies demonstrate restricted growth patterns during their NICU stays and into their childhoods (
Carroll, Slobodzian, & Steward, 2005). A meta-analysis of studies of overall brain growth demonstrated reduction in size of all major brain structures in 818 children and adolescents born at < 32 weeks or < 1,500 g when compared with 450 peers born at term (
de Kieviet, Zoetebier, van Elburg, Vermeulen, & Oosterlaan, 2012). It has also been shown that there are gender differences in the viability of ELBW infants, with females having a 1-year survival advantage (
Morse et al., 2006). The outcomes for children followed to 8 years of age who had been ELBW infants (< 1,000 g) are even worse. These children have been shown to have significantly more chronic health conditions and need for special services than normal birth weight babies; their health problems include more instances of cerebral palsy, asthma, poor vision, low IQ (< 85), poor academic skills, and poor motor skills (
Hack et al., 2011;
Hack et al., 2005;
IOM, 2007;
Mikkola et al., 2005). Predictors of cognitive impairment for ELBW babies include thrombosis of fetal vessels in the placenta, severe fetal growth restriction, male gender, and maternal obesity (
Helderman et al., 2012;
Kent, Wright, & Abdel-Latif, 2012;
Morsing, Asard, Ley, Stjerngvist, & Marsál, 2011). Predictors of mortality within 2 years after NICU discharge for ELBW babies include African American race, male gender, and prolonged NICU stay (
De Jesus et al., 2012;
Kent et al., 2012).
Consequences of PTB continue to devastate families, communities, and healthcare in general. Unfortunately, there is no cure in sight. PTB is a serious and costly health problem, affecting approximately one in eight births in the United States. It is estimated that PTBs are responsible for approximately 70% of neonatal deaths and 36% of infant deaths as well as 25% to 50% of cases of long-term neurologic impairment in children in the United States (
American College of Obstetricians and Gynecologists [ACOG], 2012c). The healthcare community is concerned about all PTBs, but the most costly births of all are those < 32 weeks. Although they make up only about 2% of all births, they result in the most devastating consequences for babies and families (
Green et al., 2005). The costs to society in the United States alone for prematurity complications were
estimated to be $26.2 billion in 2005 (
IOM, 2007). The March of Dimes commissioned a study of the cost of prematurity to employers (
Thomson Reuters, 2008). The study found that babies with a diagnosis of prematurity/LBW cost $64,713 for mother and baby compared with $15,047 for an uncomplicated birth and healthy newborn. Costs for a preterm/LBW baby were more than 10 times the cost of a normal newborn. Preterm infants are twice as likely to die by their first birthday and are more likely to suffer morbidity such as respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis than infants born at term (
March of Dimes, 2012b). Clearly, there is great need for more research about this costly public health problem to find successful methods of prevention.
LATE PRETERM BIRTHS
Most PTBs are between 34 and 36 completed weeks of gestation (
Martin et al., 2011) and do not represent infants with the most severe morbidities. However, these infants have their own set of physiologic and developmental problems, which until recently have been poorly recognized because of concentration on the dramatically severe difficulties encountered by the <32-week infants. Late preterm infants are now the focus of much research and intervention. In July 2005, the
National Institute of Child Health and Human Development (NICHD, 2005) convened a panel of experts to discuss the definition and terminology, epidemiology, etiology, biology or maturation, clinical care, surveillance, and public health aspects of “near term” PTB and “near term” infants (
Raju et al., 2006). However, the panel came to the consensus that “late preterm birth” and “late preterm infants” were better descriptors because they highlight the physiologic vulnerability of this group of preterm infants. Along with the March of Dimes, ACOG, and the American Academy of Pediatrics (AAP), the Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) was an invited participant and has been one of the professional organizations leading the way to increase awareness among healthcare providers and the public and to promote better outcomes. Before the NICHD expert panel meeting, AWHONN established its “Late Preterm Infant Initiative,” a conceptual framework for optimizing the health of these babies (
Medoff-Cooper, Bakewell-Sachs, Buus-Frank, & Santa-Donato, 2005;
Santa-Donato, 2005). This ongoing program is focused on developing evidence-based practices for caring for the subset of preterm babies born between 34 weeks and 0/7 days and 36 weeks and 6/7 days (
Raju et al., 2006).
Evaluation of the issue of late preterm infants by AWHONN (
Medoff-Cooper et al., 2005) found that these infants are often overlooked in research because they may not seem dramatically sick; they are, however, immature at birth, and have missed 4 to 6 weeks of the third trimester of gestation, putting them at risk for many health problems. There is a growing body of evidence that, compared to term babies, late preterm babies have more problems with temperature stability, feeding, hypoglycemia, respiratory distress, apnea/bradycardia, symptoms suggesting the need for sepsis evaluation, and clinical jaundice (
Medoff-Cooper et al., 2005;
Raju et al., 2006). They also are at greater risk for kernicterus, apnea, seizures, and rehospitalization (
Mally, Bailey, & Hendricks-Muñoz, 2010). It is estimated that the brain size of a fetus or a baby born at 34 to 35 weeks of gestation is only 60% of that of a baby born at term (
Raju et al., 2006), and these infants are at risk for neurologic compromise if born late preterm (
Kinney, 2006). Late preterm infants, once thought to be just lighter than full-term infants, are now known to be at higher risk for multiple morbidities, as well as infant mortality risk (
Mally et al., 2010).
Display 7-1 lists the adverse outcomes that are significantly increased in late preterm infants. Because late preterm babies reflect such a large proportion of all preterm babies, even a modest increase in the PTB rate of this group can have a significant impact on human and healthcare costs. Possible causes for the increase in late PTBs include increasing proportions of pregnant women over 35 years of age, multiple births, medically indicated births secondary to better surveillance of the mother and fetus, attempts to reduce stillbirths, stress from a variety of sources, and early elective births for convenience (
Raju et al., 2006). Late preterm infants are discussed in detail in
Chapter 21.
EARLY TERM BIRTHS
Elective births comprise a significant proportion of babies born at 37 and 38 weeks of gestation. In 2010, the March of Dimes suggested using
early term to define the subset of babies born between 37 0/7 and 38 6/7 weeks of gestation, often electively (
Fleischman, Oinuma, & Clark, 2010). The rate of early term induction rose from 2% to 8% from 1991 to 2006 in the United States, representing a 300% increase (
Murthy, Grobman, Lee, & Holl, 2011). Although it is well known that significant excess costs are associated with elective births before 37 completed weeks of gestation (
Gilbert, Nesbitt, & Danielsen, 2003), electively born early term babies also generate appreciable costs including admission to the NICU due to iatrogenic morbidity related to being born too soon (
Clark et al., 2010). When compared to babies born at 39 weeks or greater, babies born electively via cesarean at 37 or 38 weeks are at higher risk for neonatal morbidity such as adverse respiratory outcomes, mechanical ventilation, newborn sepsis, hypoglycemia, admission to the NICU, and hospitalization for 5 days or more (
Tita et al., 2009).
Clark and colleagues (2009) found similar neonatal outcomes when comparing babies electively born at 39 weeks or greater with babies electively born between 37 0/7 and 38 6/7 weeks.
There has been an emphasis on lung maturity as a determining factor in predicting risks of neonatal morbidity and mortality; however, the role of maturation of other organ systems is getting more attention (
March of Dimes, 2011). For example, the brain continues to develop throughout pregnancy, including rapid growth in the last month (
Noble, Fifer, Rauh, Nomura, & Andrews, 2012). Being born at term, but earlier than 39 weeks’ gestation, can have significant negative neurologic effects as children develop. In a study of 128,050 children who were born at term (≥ 37 weeks’ gestation), researchers linked birth data to children’s school records 8 years later and found that, even among the “normal term” range, gestational age was an important independent predictor of academic achievement (
Noble et al., 2012). Gestational age within the normal term range was significantly and positively related to reading and math scores in third grade, with achievement scores for children born at 37 and 38 weeks significantly lower than those for children born at 39, 40, or 41 weeks; this effect was independent of birth weight as well as a number of other obstetric, social, and economic factors (
Noble et al., 2012).
Elective labor inductions and elective cesarean births are preventable factors contributing to the increase in late preterm and early term births and associated costs (
March of Dimes & California Maternal Quality Care Collaborative, 2010). Evidence supports induction of labor for postterm gestation, premature rupture of membranes at term, and premature rupture of membranes near term with documented fetal lung maturity as well as significant maternal compromise such as HELLP syndrome or nonremedial fetal compromise such as fetal hydrops (
ACOG, 2009;
Mozurkewich, Chilimigras, Koepke, Keeton, & King, 2009). Other medical indications for induction before 39 completed weeks of gestation suggested by
ACOG (2009) include abruptio placentae, chorioamnionitis, fetal demise, gestational hypertension, preeclampsia, eclampsia, maternal medical conditions (e.g., diabetes mellitus, renal disease, chronic pulmonary disease, chronic hypertension, antiphospholipid syndrome), and fetal compromise (e.g., severe fetal growth restriction, isoimmunization, oligohydramnios). In 2011, further recommendations for timing of medically indicated births before 39 completed weeks of gestation were published by the NICHD based on a review of the evidence by an expert panel of perinatal clinicians and scientists (
Spong et al., 2011). See
Table 7-1 for the NICHD recommendations.
Because late preterm and early term births without medical indications have potential to result in a preventable neonatal morbidity, professional organizations have promulgated recommendations to avoid their occurrence. Estimations of the “due date” can often be miscalculated by up to 2 weeks; therefore, ACOG, AAP, and the National Institutes of Health (NIH) recommend that gestational age of 39 completed weeks of gestation be confirmed by at least one method before elective labor induction, repeat cesarean birth, or nonmedically indicated cesarean birth to avoid iatrogenic PTB (
AAP & ACOG, 2012;
ACOG, 2009;
NIH, 2006). These methods include an ultrasound measurement at less than 20 weeks of gestation that supports gestational age of 39 weeks or greater, fetal heart tones have been documented as present for 30 weeks by Doppler ultrasonography, or it has been 36 weeks since a positive serum or urine human chorionic gonadotropin pregnancy test result (
ACOG, 2009). Testing for fetal lung maturity should not be performed, and is contraindicated, when birth is required for fetal or maternal indications. Conversely, a mature fetal lung maturity test result before 39 weeks of gestation, in the absence of appropriate clinical circumstances, is not an indication for birth (
ACOG, 2008a). Adoption and consistent use of the
ACOG (2009) and NICHD (
Spong et al., 2011) guidelines for medically indicated births before 39 weeks should have a positive influence on avoidance of preventable adverse neonatal morbidity related to elective early term births.
The
American College of Nurse-Midwives (ACNM, 2010,
2012) and
AWHONN (2012) recommend awaiting spontaneous onset of labor unless there are evidence-based medical indications that outweigh the risks of induction. Awaiting spontaneous labor in the absence of medical indications for birth not only will reduce elective early term births but can also decrease the cesarean birth rate (
Fisch, English, Pedaline, Brooks, & Simhan, 2009;
Reisner, Wallin, Zingheim, & Luthy, 2009).
WHY HAS THE RATE OF PRETERM BIRTHS INCREASED?
Contributing factors to the increase in PTBs over the last three decades include increasing use of infertility treatments producing twins and higher order multiples, more births to women at older ages (>35 years of age), more medically induced prematurity (including early labor inductions), early repeat cesarean births, primary cesarean births without a medical indication, advances in maternal and fetal medicine and neonatal care (which lead both providers and patients to believe that birth at earlier gestations is not an insurmountable health hazard), more pregnancies in very-high-risk women who then require early birth, and an increase in fetal complications leading to early birth, such as IUGR (
Green et al., 2005;
Murthy et al., 2011). As some of these issues (e.g., morbidities related to late PTB, elective early labor inductions, early cesarean births) have gained more attention, the PTB rate appears to have stabilized and even decreased slightly (
Hamilton et al., 2011). It is too early to know whether this trend will continue and represent actual and sustainable improvement for this serious public health problem.
The issue of PTB of multiples and higher order multiples is particularly problematic. The twin birth rate in 2009 was 32.2 twins per 1,000 live births, a record high (
Martin et al., 2011). Of the more than 4 million U.S. live births in 2009, there were 137,217 twin births, 5,905 triplet births, 355 quadruplet births, and 80 quintuplet and higher multiples births (
Martin et al., 2011). The rate of triplet and other higher order multiple births in 2009 was 153.5 per 100,000 births. The twin birth rate increased by 76% since 1980 and by 47% since 1990 but has now stabilized, rising about 1% annually (
Martin et al., 2011). During that same time, the rate of higher order multiples rose more than 500% but has declined slightly since 2004 (
Martin et al., 2011). About 58.8% of twins are born preterm (
Martin et al., 2011). The PTB rate is 94.4% for triplets, 98.3% for quadruplets, and 97% for higher order multiples (
Martin et al., 2011). In 2006, 1% of singletons died in infancy compared with 3% of twins and 7% of triplets (
Luke & Brown, 2007). It must be remembered, however, that the high rates of PTB in the United States cannot be attributed solely to the rates of multiple births, since the preterm rate for singletons also rose 23.7% from 9.7% in 1990 to 12% in 2010 (
Hamilton et al., 2011).
PATHOPHYSIOLOGY OF PRETERM LABOR AND BIRTH
In nearly 40% of PTBs, the cause is unknown (
March of Dimes, 2011). Based on what has been learned from the research over the past few decades, it is doubtful that one causative factor for preterm labor will be discovered. All indications are that preterm labor has multiple causes, including social factors, physiologic factors, medical history factors, and illness factors (
Green et al., 2005). The March of Dimes has characterized preterm labor as a series of complex interactions of factors and pathways (
Green et al., 2005).
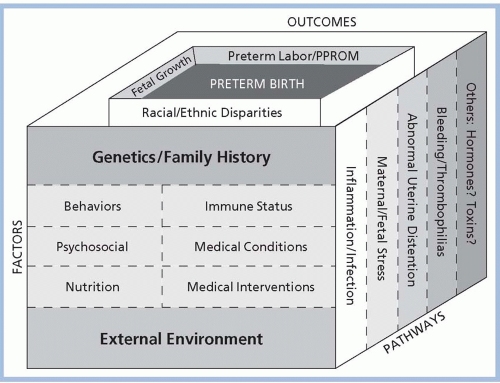
Figure 7-1 illustrates the determinants of preterm labor conceptualized by
Green and colleagues (2005). This conceptualization suggests that genetics, environment, stress, and psychosocial factors all play a role in the development of preterm labor leading to PTB. No single factor is thought to act alone; rather, factors interact in as yet unknown ways to initiate the cascade of events that result in preterm labor and birth. The external environment, including personal behaviors (e.g., smoking and drug use), psychosocial factors, nutrition, immune status, medical conditions, and medical interventions, interacts with genetics and family history through pathways that include inflammation and infection, maternal/fetal stress, abnormal uterine distention, bleeding/thrombophilia, and other possible pathways such as hormones and toxins. These factors and pathways, combined with racial and ethnic disparities, fetal growth, and preterm premature rupture of membranes, finally result in preterm labor/birth (
Muglia & Katz, 2010). When thought of in this manner, it is clear that there is no one “cause” of preterm labor and birth, and therefore, no one single “cure.” Multiple causes require research into multiple cures and preventive measures. One of the significant barriers in studying the causative factors of preterm labor and birth is the lack of knowledge regarding the exact physiologic mechanism responsible for initiation of term labor and birth. As with preterm labor, multiple theories have been proposed concerning what factors are responsible for spontaneous term labor, but none has proven conclusive.
Although research has not found the cause or causes of preterm labor, some evidence suggests avenues for further research. According to the
March of Dimes (2011), there may be four main routes leading to spontaneous premature labor:
1. Infections/Inflammation. Preterm labor is often triggered by the body’s natural immune response to certain bacterial infections, such as those involving the genital and urinary tracts and fetal membranes. Even infections far away from the reproductive organs, such as periodontal disease, may contribute to PTB.
2. Maternal or fetal stress. Chronic psychosocial stress in the mother or physical stress (e.g., insufficient blood flow from the placenta) in the fetus appears to result in production of a stressrelated hormone called corticotropin-releasing hormone. Corticotropin-releasing hormone may stimulate production of a cascade of other hormones that trigger uterine contractions and premature birth.
3. Bleeding. Placental abruption triggers the release of various proteins involved in blood clotting, which also appear to stimulate uterine contractions.
4. Uterine stretching. Overdistension of the uterus due to multiple gestation, polyhydramnios, or uterine or placental abnormalities can lead to the release of chemicals that stimulate uterine contractions.
Knowledge about these four routes may help scientists develop more effective interventions that can halt the various chemical cascades that lead to PTB (
March of Dimes, 2011).
Not all PTBs can or should be prevented. About 25% of PTBs are intentional and occur because of health problems of the mother or the fetus (e.g., IUGR, preeclampsia, abruptio placentae, pulmonary or cardiac disease); another 25% of PTBs follow rupture of membranes (a cause not currently known to be preventable). Recently, researchers have proposed reconceptualizing how we think about PTB in order to more clearly differentiate the various pathways to PTB (
Goldenberg et al., 2012;
Kramer et al., 2012;
Villar et al., 2012).
RISK FACTORS FOR PRETERM LABOR AND BIRTH
Risk factors for preterm labor and birth have been published and refined since the early 1980s (
IOM, 1985). Historically, risk factors and gestational age have defined the conversation about preterm labor and birth. However, recent international discussions suggest moving away from a gestational age-focused conceptualization of PTB to a more complex model that considers all births (including stillbirths) between 16 and 38 6/7 weeks’ gestation to be preterm and considers five components in assigning a PTB phenotype: maternal conditions, fetal conditions, placental conditions or pathology, signs of labor, and the pathway to birth (clinician initiated vs. spontaneous) (
Villar et al., 2012). This model considers only the index pregnancy and not risk factors in assigning a phenotype for PTB. It is hoped that the proposed phenotypic classification system may improve future understanding of PTB by teasing out the heterogenous causes of PTB for more focused
study. However, it will be some time before the benefits of this approach might be realized in concrete prevention strategies.
In addressing prevention of PTB, the March of Dimes suggested that there are three important known risk factors for preterm labor and birth (current multifetal pregnancy, history of a PTB, and uterine/cervical abnormalities), along with multiple categories of risk for subgroups of women (
Freda & Patterson, 2004;
March of Dimes, 2011). Additional risk factors include medical conditions predating the pregnancy, demographic factors, behavioral and environmental factors, illnesses occurring during the pregnancy, and genetics. These factors are listed in
Displays 7-3 and
7-4.
Some of the risks of preterm labor and birth have been known for decades, and some are new to the list (
IOM, 1985,
2007;
Muglia & Katz, 2010). Knowledge of the genetics of PTB (see next section) is clearly a new and evolving phenomenon, as is the risk of preterm labor after the use of assisted reproductive technologies (ARTs), proposed in a study by
Jackson, Gibson, Wu, and Croughan (2004). They performed a metaanalysis of 15 studies, examining the outcomes of singletons conceived through in vitro fertilization (n = 12,282) compared to 1.9 million spontaneously conceived singletons. Controlling for maternal age and parity, they found significantly higher odds ratios for PTB and other adverse perinatal outcomes in the singletons conceived through ART. Further risks of ART include multiple gestation, which carries a greater risk of PTB. Of live births from pregnancies conceived in ART cycles using fresh nondonor eggs or embryos in 2009, 28.9% were twins and 1.6% were higher order multiples (
Centers for Disease Control and Prevention [CDC], American Society for Reproductive Medicine, & Society for Assisted Reproductive Technology, 2011). Among ART births in 2009, 60% of twins and 97.5% of higher order multiples were born preterm, and 56.1% of twins and 92.1% of higher order multiples were LBW (
CDC et al., 2011).
Another risk that has received recent attention in the literature is periodontal disease (
Offenbacher et al., 2006). Follow-up studies of interventions in periodontal disease have attempted to identify specific interventions that are effective. A French study failed to demonstrate improved outcome with improved dental care (
Vergnes et al., 2011). A South African study identified specific periodontopathogens that, when treated together, showed improved outcome (
Africa, Kayitenkore, & Bayingana, 2010). More research is needed to further define the role and intervention in periodontal risk.
Clearly, the list of PTB risk factors is lengthy (
Goldenberg et al., 2012). A number of these risk factors are not modifiable, but some are. Some of the current thinking in this field proposes that preconception care, or care begun before a pregnancy has been conceived, could ameliorate some of these preexisting factors, such as smoking cessation, tight control of preexisting diabetes or hypertension, obesity, or low prepregnancy weight (
ACOG, 2005;
Freda, Moos, & Curtis, 2006;
Moos, 2003). The CDC held a major
conference dedicated to this topic in 2005 and has published recommendations for preconception care, all of which are aimed at reducing PTB and other poor pregnancy outcomes (
Johnson et al., 2006).
GENETIC INFLUENCES
In the late 1970s, when the topic of preterm labor and birth became prominent as an important entity for research and prevention, no thought was given to the possibility that preterm labor could have a genetic component. For decades, diligent researchers in medicine, epidemiology, public health, and nursing focused on physical symptoms, biologic pathways, and social interventions in their efforts to prevent this costly and dangerous complication of pregnancy. It was not until 2003 that the director of the National Human Genome Research Institute at the NIH announced that the sequencing of the human genome had been completed (
Lewis, 2006). This event ushered in the genomic era of health research and has impacted the study of PTB in extraordinary ways. What we once thought of as strictly a social phenomenon or a biologic accident might actually have a genetic component that could hold promise for exciting new interventions unheard of previously in history.
The possibility that preterm labor and birth has a genetic component provides us with new targets for prevention. For instance,
Wang and colleagues (2002) found that infant birth weight is particularly vulnerable in women who smoke and possess a certain gene polymorphism. This could be the answer for why some women smoke during pregnancy and give birth to normal weight babies at term, and others who smoke have preterm, small babies who are at risk for more health problems. The clinical implication is that smoking cessation could be strongly targeted toward women who smoke and have the polymorphism. This sort of tailored intervention is an example of how advances in our understanding of genetics and biomarkers could transform the way we look at PTB prevention.
It has been known for some time that women who were themselves born preterm have a higher chance of having preterm labors and births themselves (
Plunkett et al., 2009;
Ward, 2003;
Ward, Argyle, Meade, & Nelson, 2005). Is it possible that these women and their sisters and mothers have a genetic predisposition toward PTB? If so, can it be altered? More genetic research on this topic may answer these questions.
Genetic polymorphisms have been implicated in PTB (
Kalish, Vardhana, Normand, Gupta, & Witkin, 2006).
Giarratano (2006) described the effect of genetics on PTB and provided information about the genes that could possibly be implicated in preterm labor and birth. These data are reproduced here as
Tables 7-2,
7-3 and
7-4. These genes control pro-inflammatory cytokines (involved in possible infection and preterm premature rupture of membranes), the labor cascade genes (being studied for their role in the initiation of labor), and the vasculopathic genes (involved in vascular problems such as preeclampsia and thrombophilias). None of these lines of genetic inquiry has reached maturity, but they have given researchers entirely new areas in which to discover more information about how preterm labor begins, and how, possibly, to avoid it.