Models of Care
Pamala D. Larsen
INTRODUCTION
As the population age 65 and older increases and the healthcare system sees more individuals with chronic illness, healthcare providers and third-party payers are examining how to care for individuals with chronic disease long term. Increasingly we hear about disease management models that have demonstrated better patient outcomes than “usual care.” Less well publicized, however, is nursing’s role in caring for individuals with chronic disease. For some of us, it has always made sense that chronic care should be nursing’s domain, particularly as the profession looks at care as opposed to cure.
The United States continues to outspend similar nations in health care. As noted in Chapter 1, however, more money does not translate into better patient outcomes or better value for the consumer. Phrases heard today are “value-based” and “value-added”—whether in manufacturing, architecture, engineering, or now health care. What is the value of the health care that the patient is receiving? Robinson (2008) defines value in health care as measured in terms of contributions of health care minus the attendant costs, with costs and contributions conceptualized broadly (p. 11).
As of March 2010, the United States has a new healthcare reform bill in place. Will it be successful? Will it make a difference? Does it address the “real” issues of our healthcare system? There are already calls to repeal the bill after only a year in existence. Clearly, health care is at a crossroads with or without the Affordable Care Act (ACA) and at the core of that crossroads is chronic care. How do we effectively provide quality care to those with chronic conditions?
This chapter provides an overview of models and frameworks that provide care for individuals with chronic illness and their families.
Historical Perspectives
In 1983, diagnosis-related groups (DRGs) for reimbursement were initiated for all Medicare patients. This was the direct result of trying to find a better way to pay care providers than the retrospective payment system that was in place from 1965 to 1983. Furthermore, healthcare costs were escalating and it seemed that paying care providers prospectively, per diagnosis group, would decrease costs. DRGs were implemented using the International Classification of Diseases, Ninth Revision, Clinical Modifications (ICD-9-CM). ICD-9-CM coding classified diseases, symptoms, and procedures with individual codes. Although DRGs were used initially only for Medicare patients, third-party payers use the coding (now in its 10th revision with plans for an 11th in 2015) as well.
With the advent of DRGs, acute care facilities soon developed clinical pathways or care algorithms for patients with a diagnosis that matched a specific DRG. Patients with heart failure, myocardial infarction, appendectomy, cholecystectomy, stroke, diabetes, and so forth were placed within standard care plans, care maps, clinical pathways, or algorithms (the name varied in each institution) as a way to monitor these patients and make sure they were “on track” for discharge. Because the hospital was to be paid a certain amount of money for each individual with a specific condition, it was critical that patients were treated swiftly and effectively and discharged in a timely manner. Although one would not consider DRGs a disease or illness management model, this change to Medicare in the 1980s has influenced how we manage care today.
IMPACT
The direct and indirect costs associated with providing appropriate care for someone with multiple chronic conditions cannot be overstated (see Chronicity, Chapter 1). Fully 23% of Medicare beneficiaries with five or more chronic conditions account for 68% of the program’s funding (Anderson, 2005). From a cost perspective alone, the need to provide high quality care efficiently hailed the advent of both formal and informal disease management programs. Disease management programs may originate from federal and state agencies as well as from for-profit and not-for-profit companies.
Disease Management Versus Illness Management
The majority of models available today for patient care are disease management models. These models monitor the physiologic markers of disease, the measurement of one’s glycosylated hemoglobin (HbA1c), the forced expiratory volume (FEV) of a patient with chronic obstructive pulmonary disease (COPD), the number of medications prescribed to a patient, the number of visits to the healthcare provider, and so forth. However, looking at the disease, the pathophysiology, and the required medications is only one part of caring for the patient, and, quite frankly, that is the easier part—the measurable part. The illness experience of an individual patient, the uniqueness of the patient, and the patient’s living situation, social support, and coping mechanisms —whether effective or ineffective—are the other components of the patient’s life that disease management programs do not address.
INTERVENTIONS
During the mid to late 1990s, many disease management companies were formed, with most having the goal of providing cost-effective care to those with chronic conditions. By 1999 there were 200 companies nationwide offering disease management services for such conditions as diabetes, asthma, and heart failure (Bodenheimer, 2003). Most of these programs did not originate within healthcare institutions but were outsourced to outside firms. Today, few of those companies exist or are profitable, primarily because their focus was on one specific disease, when typically the older adult population has multiple chronic conditions. As an example, fewer than one half of the rehospitalizations among patients initially hospitalized with heart failure are actually attributed to heart failure. The other hospitalizations are related to conditions that predispose to heart failure such as coronary artery disease, hypertension, COPD, and
so forth (DeBusk, West, Miller, & Taylor, 1999). Disease management companies offered programs that were just that, programs, with neither a systems approach nor an integration of these programs into a healthcare system or institution. In addition, a number of those disease management programs were based on physician specialty practice and not primary care. As we look at older adults, they may have several chronic conditions necessitating going to several different specialty physicians. Therefore, programs based on specialty practice typically did not work.
so forth (DeBusk, West, Miller, & Taylor, 1999). Disease management companies offered programs that were just that, programs, with neither a systems approach nor an integration of these programs into a healthcare system or institution. In addition, a number of those disease management programs were based on physician specialty practice and not primary care. As we look at older adults, they may have several chronic conditions necessitating going to several different specialty physicians. Therefore, programs based on specialty practice typically did not work.
Most of the literature today looks at disease management models versus illness management models. However, in most studies, the definition of disease management and the components of each program vary, making it hard to compare programs and health outcomes of participants. When performing a meta-analysis or systematic review, it becomes difficult to figure out inclusion criteria for studies, because each program is different. Furthermore, when looking at outcomes, what specific component of the program “makes a difference” in the health outcome or is it the combination of components acting interdependently?
Mattke, Seid, and Ma (2007), in their analysis of disease management programs, suggest in broad terms that disease management refers to a system of coordinated healthcare interventions and communications to help patients address chronic disease and other health conditions. Disease management programs are “big business,” with 96% of the top 150 U.S. payers offering some form of disease management service and 83% of more than 500 major U.S. employers using programs to help individuals manage their health (as cited in Mattke et al., 2007, p. 670). Revenues associated with these programs have grown significantly from $78 million in 1997 to nearly $1.2 billion in 2005 and projected to top $1.8 billion by the end of 2008 (Mattke et al., 2007). What are the health outcomes of spending $1 to 2 billion a year? Are these programs making a difference in health outcomes, and, if so, are they reducing costs in other areas?
In their review of three evaluations of large-scale, population-based programs, 10 meta-analyses, and 16 systematic reviews covering 317 studies, Mattke and colleagues (2007) found consistent evidence of improved processes of care and disease control, but no conclusive support of improved health outcomes. In addition, when the costs of the programs and/or interventions were accounted for and then cost savings subtracted, there was no evidence of a net reduction in medical costs (pp 675-676).
TRICARE Management Activity, who administers healthcare benefits for U.S. military service personnel, retirees, and their dependents, developed a disease management program for beneficiaries with diabetes. A quasi-experimental approach assessed the program’s impact for 37,370 beneficiaries ages 18 to 64 living in the United States. Beneficiaries were categorized as “uncontrolled” or “controlled” based on past medical claims. The study compared observed outcomes to predicted outcomes in the absence of diabetes management. Results indicated that total annual medical savings per participant averaged $783. More active participation in the program was associated with lower medical costs (Dall et al., 2010).
Buntin Jain, Mattke, and Lurie (2009) suggest that results from disease management programs may be skewed because of selection bias. Selection bias includes patients being recruited into programs because they are likely to attain quality and cost benefits (typically the more
engaged patient interested in self-management). The conundrum is whether the disease management program itself causes the results or whether it is the selection of the appropriate patients that make the difference (Buntin et al., 2009).
engaged patient interested in self-management). The conundrum is whether the disease management program itself causes the results or whether it is the selection of the appropriate patients that make the difference (Buntin et al., 2009).
Chronic Care Model
The best known model for providing care to those with chronic disease is the chronic care model (CCM). Work on this model began in the early 1990s with Dr. Edward Wagner, an internist and director of the Seattle-based MacColl Institute for Healthcare Innovation at the Center for Health Studies, Group Health Cooperative. Wagner identified three issues in providing care to those with chronic illness through primary care (Wielawski, 2006):
Primary care offices are set up to respond to acute illnesses rather than anticipate and respond proactively to patients’ needs (which is what individuals with chronic illness need).
Patients with chronic illness are not adequately informed about their conditions and they are not supported in the self-care of their conditions beyond the physician’s office.
Physicians are too busy to educate and support patients with chronic illness to the degree needed for them to stay healthy. (p. 5)
Wagner’s (1998) solution was to replace the physician-centered office with a structure that supported a team of professionals that collaborated with the patient in his or her care. Early implementation of his model took place with 15,000 patients with diabetes at the Group Health Cooperative, a 590,000-member health maintenance organization in Seattle. During 5 years, the percentage of patients with up-todate screening improved; blood sugar levels and the regularity of monitoring improved; patients reported higher satisfaction with their care; and admission to acute care facilities decreased.
During this period of time in the mid to late 1990s, Wagner and associates partnered with The Robert Wood Johnson Foundation (RWJ) to further develop the model. The model was refined and published in its current form in 1998. Improving Chronic Illness Care (2006-2011), a national program through RWJ, was launched in 1998 with the CCM as its core (Figure 19-1). The CCM is not a model for individual care, but for large populations of individuals. It does not redesign patient care, but redesigns clinical practices that are delivering care by implementing system and process change.
A 2009 Intervention Review of an earlier Cochrane Review supported the use of the CCM with clients with both type I and type II diabetes. Forty-one studies with a total of 48,000 clients were involved in the review. Renders and colleagues (2000) concluded that multifaceted professional interventions can enhance the performance of healthcare professionals in managing clients with diabetes. Although using the model enhanced process outcomes, the effect on client health outcomes was less clear.
Studies from 2000 through 2009 were reviewed to determine the impact of the CCM in redesigning care. For this review, a CCM-based intervention was defined as an intervention that integrated changes that involved most or all of the six areas of the model: self-management support, decision support, delivery system design, clinical information systems, healthcare organization, and community resources (Coleman, Austin, Brach, & Wagner, 2009). Eighty-two
studies were retained for the final study. Published evidence suggests that practices redesigned in accord with the CCM generally improve the quality of care and the outcomes for patients with various chronic illnesses (Coleman et al., 2009, p. 81).
studies were retained for the final study. Published evidence suggests that practices redesigned in accord with the CCM generally improve the quality of care and the outcomes for patients with various chronic illnesses (Coleman et al., 2009, p. 81).
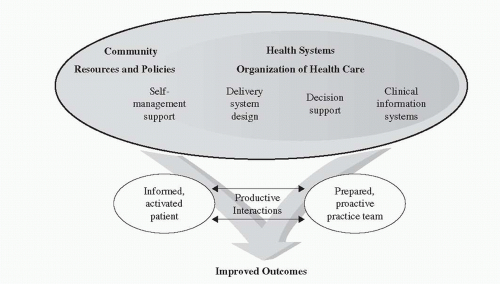 FIGURE 19-1 The Chronic Care Model. Source: Wagner, E.H. (1998). Chronic disease management: What will it take to improve care for chronic illness? Effective Clinical Practice, 1, 2-4. |
Although the CCM is a model for primary medical care, Jacelon, Furman, Rea, Macdonald, & Donaghue (2011) have adapted the model for long-term care. The six constructs of the CCM were implemented to create a model for highquality chronic disease care.
Guided Care
Using the CCM as a basis, researchers at Johns Hopkins University developed a care model, Guided Care, to improve the quality of life and efficiency of resource utilization for older adults with multiple chronic conditions. Guided Care enhances the use of primary care (versus specialty care) and utilizes the following seven principles of chronic care: disease management; self-management; case management; lifestyle modification; transitional care; caregiver education and support; and geriatric evaluation and management (Boyd et al., 2007, p. 697). What is unique about the Guided Care model is the use of registered nurses specially trained in Guided Care concepts, who, in turn, use a computerized electronic health record (EHR) in working with two to five primary care physicians to meet the needs of 50-60 older adults with multiple comorbidities. The Guided Care Nurse (GCN) is based in the primary care physician’s office, and performs eight clinical activities, guided by scientific evidence and the patients’ priorities (p. 698). Pilot versions of Guided Care included several of the eight core activities. The first major application of guided care occurred in a
cluster-randomized controlled trial (RCT) through Johns Hopkins University. The study utilized 8 sites (49 physicians) in the Baltimore-Washington, DC area, with 904 patients in either the experimental group of Guided Care or the control group (usual care) (Boult et al., 2008). Patients eligible for the study were those 65 years old or older and ranking in the upper quartile of risk for using health services during the coming year. The eight clinical activities performed by the GCN included:
cluster-randomized controlled trial (RCT) through Johns Hopkins University. The study utilized 8 sites (49 physicians) in the Baltimore-Washington, DC area, with 904 patients in either the experimental group of Guided Care or the control group (usual care) (Boult et al., 2008). Patients eligible for the study were those 65 years old or older and ranking in the upper quartile of risk for using health services during the coming year. The eight clinical activities performed by the GCN included:
1. Assessment. Initial assessments include medical, functional, cognitive, affective, psychosocial, nutritional, and environmental. Other tools used may include the Geriatric Depression Scale and the CAGE alcoholism scale. The client is also asked what his or her priorities are for improved quality of life.
2. Planning. The EHR merges the assessment data with evidence-based practice guidelines to create a preliminary care guide that manages and monitors the patients’ health conditions. The GCN and the primary care physician then personalize the care guide with input from the patient and family. The end result is a patient-friendly version of the plan called “My Action Plan,” written in lay language and given to the patient.
3. Chronic disease self-management (CDSM). The GCN encourages the patient’s self-efficacy in the management of his or her chronic conditions. The patient is referred to a free, local 15-hour CDSM course led by trained lay people and supported by the GCN. In this program—developed by Kate Lorig and associates at Stanford University—the patient learns how to operationalize the action plan.
4. Monitoring. The GCN monitors each patient at least monthly by telephone to address issues promptly. The EHR plays an important role in the monitoring by providing reminders about each patient (Boyd et al., 2007).
5. Coaching. Motivational interviewing is used to facilitate the patient’s participation in care along with reinforcing adherence to the action plan (Boyd et al., 2007). The GCNs are trained in motivational inter-viewing principles and strategies to assist in this process.
6. Coordinating transitions between sites and providers of care. The GCN is the primary coordinator of care for patients in this program, and is thus responsible for the care transitions that occur between home, the emergency room, hospitals, long-term care facilities, and other care settings.
7. Educating and supporting caregivers. The GCN works with family or other unpaid caregivers of the patients to educate and support them. This may include individual or group assistance, support group meetings, or ad hoc telephone consultation (Boyd et al., 2007).
8. Accessing community resources. Deter-mining appropriate community resources for the patient, such as Meals on Wheels, transportation needs, and so forth are key functions of the GCN. The idea is not to duplicate services, but to utilize the services available in the community.
In April 2008, 6 months into the RCT, data suggested that the Guided Care model provided improved quality of care, reduced medical care costs, and there was high satisfaction in both the primary care physicians and the GCNs (Boult et al., 2008). Based on these early results, two of the
managed care partners in the trial, Kaiser Permanente and Johns Hopkins HealthCare, agreed to continue to pay the costs of the GCNs for an additional year. However, 18-month outcomes demonstrated few results. The study looked at the use of health services and included 850 older patients at high risk for using healthcare in the future. The only statistically significant overall effect of guided care was a reduction in episodes of home health care (Boult et al., 2011).
managed care partners in the trial, Kaiser Permanente and Johns Hopkins HealthCare, agreed to continue to pay the costs of the GCNs for an additional year. However, 18-month outcomes demonstrated few results. The study looked at the use of health services and included 850 older patients at high risk for using healthcare in the future. The only statistically significant overall effect of guided care was a reduction in episodes of home health care (Boult et al., 2011).
Program of All-Inclusive Care for the Elderly (PACE)
Although the Program of All-Inclusive Care for the Elderly (PACE) was not specifically developed for individuals with chronic illness, it is obvious that the majority of older adults accessing this program could have at least one chronic condition. PACE is a capitated benefit authorized by the Balanced Budget Act (BBA) of 1997 that offers comprehensive health care to older adults. PACE is modeled after the successful On Lok Senior Health Services program in San Francisco. The On Lok model showed successful outcomes in a number of demonstration projects funded through the Centers for Medicare & Medicaid Services (CMS), then known as the Health Care Financing Administration (HCFA), in the 1980s and 1990s. PACE is a permanent entity within the Medicare program that enables states to provide PACE services to Medicaid beneficiaries as a state option.
Participants in PACE must be 55 years of age, live in a PACE service area, and be certified as eligible for nursing home care. The program allows most of its participants to receive services while they continue to live at home. Capitated financing allows care providers to deliver all services that the participants need, rather than those that are limited under Medicare and Medicaid fee-for-service systems (www.cms.hhs.gov/pace/). PACE becomes the sole source of services for the Medicare and Medicaid eligible enrollees. As of 2011 there were 58 PACE providers located in 26 states.
Mukamel and colleagues (2007) attempted to determine what program characteristics of PACE were associated with the risk-adjusted health outcomes of mortality, functional status, and self-assessed health. The research examined 3,042 newly enrolled persons in 23 PACE programs over a 4-year period (1997 to 2001). There were a number of program characteristics that were significantly associated with better functional outcomes. These included: a medical director who was a trained geriatrician; medical directors who spent time providing direct patient care; programs with effective interdisciplinary teams; teams composed of more aides than professionals; the same ethnicity of participant and team member; and larger and older PACE programs (Mukamel et al., 2007).
Fewer program characteristics were associated with participant self-assessed health outcomes. Higher staffing levels, having more diverse services, and having a match between the ethnicity of the participant and the staff member were associated with higher self-assessed health outcomes (Mukamel et al., 2007, p. 524).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


