Metabolic Alkalosis
QUICK LOOK AT THE CHAPTER AHEAD
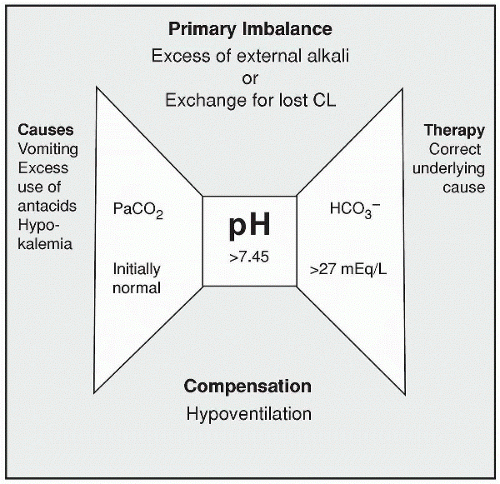
Metabolic alkalosis occurs when the bicarbonate level rises above 27 mEq/L and the pH above 7.45. It may result from an excess of HCO3–, a deficit of acid, or a combination of both. Here we discuss the causes, manifestations, and treatment of metabolic alkalosis.
Metabolic alkalosis occurs when the bicarbonate level rises above 27 mEq/L and the pH above 7.45. It may result from an excess of HCO3–, a deficit of acid, or a combination of both. The body’s production and reabsorption of bicarbonate are usually maintained in a balance so that alkalosis does not occur. However, an intake of excess bicarbonate through antacids or overuse of bicarbonate products such as parenteral solutions containing lactate, hyperalimentation, or citrate with blood transfusions can increase the bicarbonate level above 27 mEq/L and move the pH above 7.45.
 Metabolic alkalosis occurs when the body loses too much acid, such as severe situations of gastric suction, vomiting, binge-purge, or use of diuretics. Metabolic alkalosis can also occur when too much base is absorbed, such as with overuse of antacids or products containing bicarbonate.
Metabolic alkalosis occurs when the body loses too much acid, such as severe situations of gastric suction, vomiting, binge-purge, or use of diuretics. Metabolic alkalosis can also occur when too much base is absorbed, such as with overuse of antacids or products containing bicarbonate.Another possible cause is the removal of H+ and Cl– from the stomach through emesis
or gastric suction, resulting in an excess of base. In situations where K+ is lost, such as through the use of diuretics or metabolic disorders, H+ excretion is increased as the kidneys work to conserve K+. The body also shifts the hydrogen ion into the cell during conditions of hypokalemia and increases renal excretion of acid, contributing to or causing metabolic alkalosis.
or gastric suction, resulting in an excess of base. In situations where K+ is lost, such as through the use of diuretics or metabolic disorders, H+ excretion is increased as the kidneys work to conserve K+. The body also shifts the hydrogen ion into the cell during conditions of hypokalemia and increases renal excretion of acid, contributing to or causing metabolic alkalosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree